Contact ion pairs on a protonated azamacrocycle
advertisement

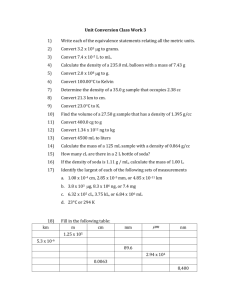
Caterina Fraschetti, Antonello Filippi, Maria Elisa Crestoni, Enrico Marcantoni, Marco Glucini, Laura Guarcini, Maria Montagna, Leonardo Guidoni, Maurizio Speranza* Contact ion pairs on a protonated azamacrocycle: the role of the anion basicity. Supplementary Material Experimental Details. Figure S1. ESI-MS spectrum of methanolic solutions containing 10 -5 M alkali chlorides + 10-5 M of M. Figure S2. ESI-MS spectrum of methanolic solutions of M (10-5 M) + acetic acid (10-5 M). Figure S3. ESI-MS spectrum of methanolic solutions of M (10-5 M) + naproxen (10-5 M). Figure S4. ESI-MS spectrum of methanolic solutions of M (10-5 M) + sodium acetate (10-5 M). Figure S5. ESI-MS spectrum of methanolic solutions of M (10-5 M) + ammonium acetate (10-5 M). Figure S6. CID spectrum of the [M•H•CF3COOH]+ (m/z 587.0) complex yielding the [M•H•FH]+ (m/z 493.1) daughter by the formal loss of the CF2COO fragment (collision energy 40 eV lab frame). Figure S7. Proton-proton and proton-K+ distances in the B3LYP/6-31G*-calculated most stable structures of the [M•H2]2+ and [M•H•K]2+ dications. Figure S8. Time-dependent relative abundances of [M•K•HF]+ (m/z 493) and [M•K•CD3COOH]+ (m/z 536) from the reactions between CD3COOH and the [M•K•HF]+ complex. Figure S9. Time-dependent relative abundances of [M•K•H35Cl]+ (m/z 509), [MDx•K•H35Cl]+ (m/z 510-513; x=1-3), and [MDx•K•CD3COOH]+ (m/z 536-540) from the reactions between CD3COOH and the [M•K•H35Cl]+ complex. At t ≤ 20 s, the [MD3•K•CD3COOH]+ product (m/z 541) is barely detectable. Experimental Details. Materials. The racemate of the hexaazamacrocycle M was synthesized according to the reaction sequence of Scheme 1. Scheme S1. Hexaazamacrocycle M synthetic sequence. The macrocyclization between a racemic (±)-trans-1,2-diaminocyclohexane (3) and 2,6-pyridinecarboxaldehyde (2) was carried out in order to obtain the [2+2] macrocycle 4 of interest. High dilution conditions in a protic polar solvent such as methanol, together with the use of starting materials in a stoichiometric ratio 1:1, promote the cyclization reaction rather than the formation of oligomers. The use of the highly polar solvent, in fact, allows the precipitation of the cyclized products, much less polar of the starting substrates, shifting the equilibrium toward their formation. [1] Once the macrocyclic imine 4 was formed, it was able to undergo reduction to the corresponding macrocyclic amine M, which is more stable towards hydrolytic decomposition. The reduction can be carried out using several reagents, but sodium borohydride has provided the best results, and was directly added to the reaction mixture after macrocyclization step. The success of this strategy requires the necessity of subjecting the mixture of the reaction of macrocyclization in a period at reflux to convert totally the side product [3+3] (5) in [2+2] product (M). It is known that the [3+3] cyclocondensation product is formed under kinetic control, while [2+2] cyclocondensation product points toward this macrocycle as the product of thermodynamic control. [2] Synthesis of 2,6-pyridinedicarboxaldehyde (2). To a 100 mL round-bottom flask and under nitrogen atmosphere were added 2,6-pyridinedimethanol (1.25 g, 9.0 mmol), anhydrous 1,4-dioxane (46 mL) and in three portions SeO2 (1 g, 9.0 mmol). The reaction mixture was stirred at reflux until the disappearance of starting material (3 h, checked by TLC analysis). The hot reaction mixture was filtered through a short plug of Celite to remove deposited selenium metal, and the residue was washed with hot (about 55°C) 1,4-dioxane. The filtrate was concentrated under vacuum and the obtained crude product was purified by silica gel column chromatography using hexane-ethyl acetate (8:2) as an eluent to give 1.05 g (yield 82%) of the dialdehyde 2: IR (neat, cm-1) 1721; 1H NMR 8.04-8.08 (m, 1H), 8.15 (d, 2H, J = 7.27 Hz), 10.15 (s, 1H); 13C NMR 124.9, 133.4, 153.6, 192.2; EI-MS m/z 135 (M+), 107 (100%), 106, 79, 78, 63, 52, 38; Anal. Calcd for C7H5NO2: C, 62.22; H, 3.73; N, 10.37. Found: C, 62.16; H, 3.69; N, 10.35. Synthesis of 18 membered hexaazamacrocycle reacemate (M). A solution of 2 (0.45 g, 3.3 mmol) in methanol (5 mL) was added drop-wise to a stirred solution of diamine 3 (0.381 g, 3.3 mmol) in methanol (45 mL) and the reaction mixture was refluxed for 3 h. Then the reaction mixture was cooled to room temperature and toluene (50 mL) was added to form a mixture MeOH-toluene 1:1. NaBH4 (0.21 g, 5.35 mmol) was added in three portions and the reaction mixture was stirred at room temperature for 24 h. The crude reaction was concentrated under reduced pressure and the residue was dissolved in water. Then 5% mol NaOH was added to the solution till pH becomes 13. Extraction with chloroform and the extracts were then washed with brine and dried over Na 2SO4. The concentration under vacuum gives the pure desired M (0.5 g, yield 70%): 1H NMR 0.97-1.04 (m, 4H), 1.13-1.18 (m, 4H), 1.68 (d, 4H, J = 8.55 Hz), 2.11-2.18 (m, 4H), 2.68 (s, 4H), 3.73 (d, 4H, J = 14.10 Hz), 3.96 (d, 4H, J = 13.68 Hz), 6.98 (d, 4H, J = 7.69 Hz), 7.49 (t, 2H, J = 7.69 Hz); 13C NMR 24.9, 32.8, 51.5, 59.5, 121.4, 136.9,160.3; ESI-MS m/z 435 [M+H], 457 [M+Na], 218 [M+2H]; Anal. Calcd for C26H38N6: C, 71.85; H, 8.81; N, 19.34. Found: C, 71.84; H, 8.79; N, 19.35. [1] J. Gregoliński, J. Lisowski, L. Tadeusz, Org. Biomol. Chem. 2005, 3161-3166. [2] N. Kuhuert, G. M. Rossignolo, A. Lopez-Periago, Org. Biomol. Chem. 2003, 1157-1170. Figure S1. ESI-MS spectrum of methanolic solutions containing 10-5 M alkali chlorides + 10-5 M of M. Figure S2. ESI-MS spectrum of methanolic solutions of M (10-5 M) + acetic acid (10-5 M). Figure S3. ESI-MS spectrum of methanolic solutions of M (10-5 M) + naproxen (10-5 M). Figure S4. ESI-MS spectrum of methanolic solutions of M (10-5 M) + sodium acetate (10-5 M). Figure S5. ESI-MS spectrum of methanolic solutions of M (10-5 M) + ammonium acetate (10-5 M). Figure S6. CID spectrum of the [M•H•CF3COOH]+ (m/z 587.0) complex yielding the [M•H•FH]+ (m/z 493.1) daughter by the formal loss of the CF2COO fragment (collision energy 40 eV lab frame). Figure S7. Proton-proton and proton-K+ distances in the B3LYP/6-31G*-calculated most stable structures of the [M•H2]2+ and [M•H•K]2+ dications. 100 90 ion abundance (%) 80 m/z 493 70 60 50 40 30 m/z 536 20 10 0 0.0 0.2 0.4 0.6 0.8 1.0 1.2 1.4 1.6 1.8 2.0 time (s) Figure S8. Time-dependent relative abundances of [M•K•HF]+ (m/z 493) and [M•K•CD3COOH]+ (m/z 536) from the reactions between CD3COOH and the [M•K•HF]+ complex. 100 90 m/z 509 ion abundance (%) 80 m/z 510-512 70 60 50 m/z 536-540 40 30 20 10 0 0 2 4 6 8 10 12 14 16 18 20 time (s) Figure S9. Time-dependent relative abundances of [M•K•H35Cl]+ (m/z 509), [MDx•K•H35Cl]+ (m/z 510513; x=1-3), and [MDx•K•CD3COOH]+ (m/z 536-540) from the reactions between CD3COOH and the [M•K•H35Cl]+ complex. At t ≤ 20 s, the [MD3•K•CD3COOH]+ product (m/z 541) is barely detectable.