Abstract-Buxadera-JOINT-COST-Bertinoro-13-17-September
advertisement
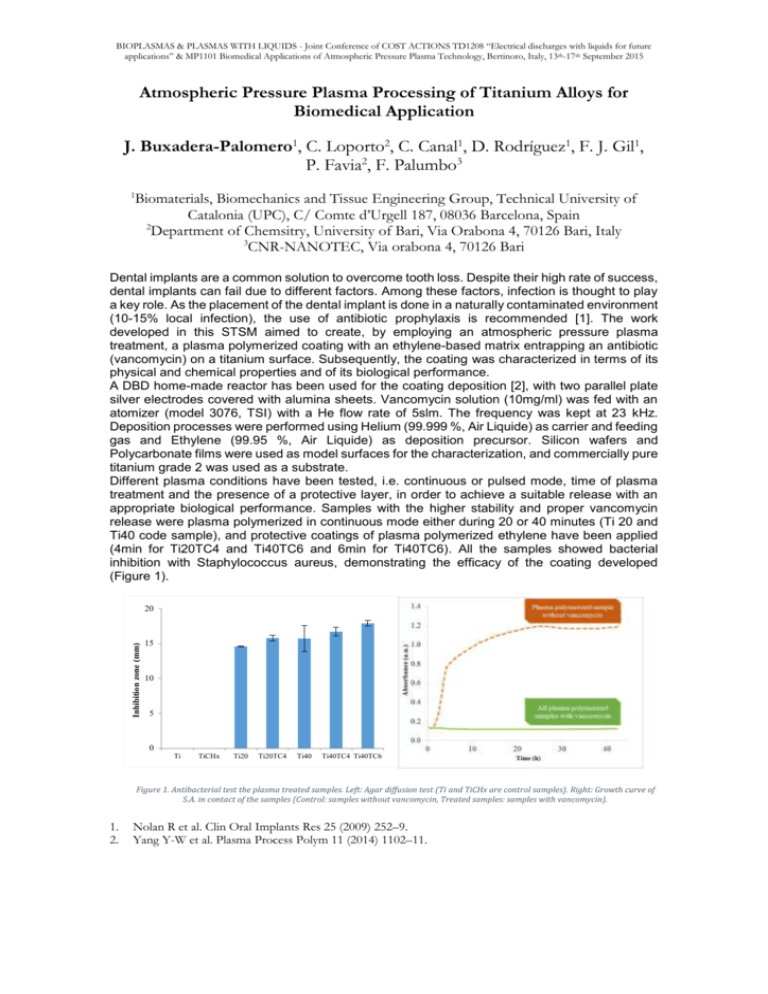
BIOPLASMAS & PLASMAS WITH LIQUIDS - Joint Conference of COST ACTIONS TD1208 “Electrical discharges with liquids for future applications” & MP1101 Biomedical Applications of Atmospheric Pressure Plasma Technology, Bertinoro, Italy, 13th-17th September 2015 Atmospheric Pressure Plasma Processing of Titanium Alloys for Biomedical Application J. Buxadera-Palomero1, C. Loporto2, C. Canal1, D. Rodríguez1, F. J. Gil1, P. Favia2, F. Palumbo3 1 Biomaterials, Biomechanics and Tissue Engineering Group, Technical University of Catalonia (UPC), C/ Comte d’Urgell 187, 08036 Barcelona, Spain 2 Department of Chemsitry, University of Bari, Via Orabona 4, 70126 Bari, Italy 3 CNR-NANOTEC, Via orabona 4, 70126 Bari Dental implants are a common solution to overcome tooth loss. Despite their high rate of success, dental implants can fail due to different factors. Among these factors, infection is thought to play a key role. As the placement of the dental implant is done in a naturally contaminated environment (10-15% local infection), the use of antibiotic prophylaxis is recommended [1]. The work developed in this STSM aimed to create, by employing an atmospheric pressure plasma treatment, a plasma polymerized coating with an ethylene-based matrix entrapping an antibiotic (vancomycin) on a titanium surface. Subsequently, the coating was characterized in terms of its physical and chemical properties and of its biological performance. A DBD home-made reactor has been used for the coating deposition [2], with two parallel plate silver electrodes covered with alumina sheets. Vancomycin solution (10mg/ml) was fed with an atomizer (model 3076, TSI) with a He flow rate of 5slm. The frequency was kept at 23 kHz. Deposition processes were performed using Helium (99.999 %, Air Liquide) as carrier and feeding gas and Ethylene (99.95 %, Air Liquide) as deposition precursor. Silicon wafers and Polycarbonate films were used as model surfaces for the characterization, and commercially pure titanium grade 2 was used as a substrate. Different plasma conditions have been tested, i.e. continuous or pulsed mode, time of plasma treatment and the presence of a protective layer, in order to achieve a suitable release with an appropriate biological performance. Samples with the higher stability and proper vancomycin release were plasma polymerized in continuous mode either during 20 or 40 minutes (Ti 20 and Ti40 code sample), and protective coatings of plasma polymerized ethylene have been applied (4min for Ti20TC4 and Ti40TC6 and 6min for Ti40TC6). All the samples showed bacterial inhibition with Staphylococcus aureus, demonstrating the efficacy of the coating developed (Figure 1). Inhibition zone (mm) 20 15 10 5 0 Ti TiCHx Ti20 Ti20TC4 Ti40 Ti40TC4 Ti40TC6 Figure 1. Antibacterial test the plasma treated samples. Left: Agar diffusion test (Ti and TiCHx are control samples). Right: Growth curve of S.A. in contact of the samples (Control: samples without vancomycin, Treated samples: samples with vancomycin). 1. 2. Nolan R et al. Clin Oral Implants Res 25 (2009) 252–9. Yang Y-W et al. Plasma Process Polym 11 (2014) 1102–11.