Card - Ingegneria Chimica
advertisement

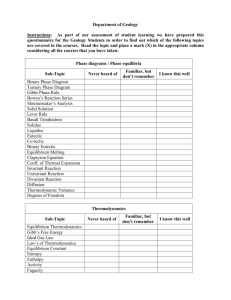
PRINCIPLES OF CHEMICAL ENGINEERING 2 Mod. THERMODYNAMICS IN NON IDEAL SYSTEMS Program of the course. Fugacity in gas and liquid mixtures. Excess properties and activity coefficients. Estimate of activity coefficients for binary and multicomponent systems. Duhem Margules equation. Tests for thermodynamic consistency. Fugacity coefficients for pure component and in mixture. State equations. The law of corresponding states: two and three parameter equations. Thermodynamic properties from volumetric data. Estimation of fugacity coefficient. Departure functions. Some numerical examples. Fluid phase equilibrium in non-ideal binary and multicomponent systems. Some numerical examples. Reaction equilibrium for non-ideal systems. Requirements. It is anticipated that students will have a solid grounding in mathematics, chemistry, physics, thermodynamics of ideal system to be able to deal with the subject matter, but no formal prerequisite is required. Typology of the activities. An oral exam and a discussion and evaluation of numerical exercises performed independently by the student.