CHML212
advertisement

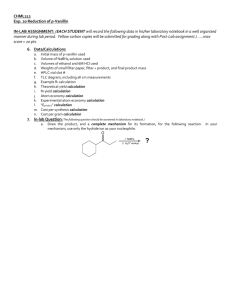
CHML212 Exp. 24 Synthesis and GC Analysis of FAMEs IN-LAB ASSIGNMENT: (EACH STUDENT will record the following data in his/her laboratory notebook in a well organized manner during lab period. Yellow carbon copies will be submitted for grading along with Post-Lab assignment.) …..max score = 20 pts. 6. Data/Calculations a. b. c. d. e. f. Unknown used TLC diagram with all cm measurements TLC solvent system used TLC detection method used GC vial slot number All adjusted area % calculations 7. In-lab Question (The following question should be answered in laboratory notebook.) a. Draw the structures of the 3 fatty acid methyl esters which would result from the following transesterification: A. B. CH2O C. D. CHO E. F. CH2O G. O C CH2(CH2)4CH3 O C CH2(CH2)8CH3 O C CH2(CH2)6CH3 KOH CH3OH CHML212 Exp. 24 Synthesis and GC Analysis of FAMEs POST-LAB ASSIGNMENT: (EACH LAB GROUP will submit one copy of a typewritten, paragraph style report addressing all of the points listed below. Must be written using PAST TENSE, PASSIVE VOICE. ) …..max score = 50 pts. 8. Experimental (Write 1-2 paragraphs including all of the following. Do NOT present a bulleted outline.) What type of reaction did you perform? Describe the actual synthetic procedure. Include names of any reactants used and desired product, as well as name of solvent and catalyst used (if any). Be sure to give actual names and volumes/masses of compounds used during the synthesis (not just what the lab manual tells you to use). Describe the purification technique used to isolate the desired product, including actual names and volume/mass of any compounds used during purification process. Describe the analytical technique used to evaluate the product, including actual names and volume/mass of any compounds used during sample preparation and analysis. 9. Results (Cut and paste completed tables into your document.) Table 24.1 TLC results TLC Diagram (Show measurements for all spots and solvent front in cm) Compound Table 24.2 GC results GC Retention Times (min) Standard Sample Area Percent Adjusted Area Percent Pentane Methyl Palmitate Methyl Linolate Methyl Oleate Methyl Stearate Fatty Acid Type % SFA % MUFA % PUFA Table 24.3 Expected vs. Experimental Results Expected Experimental Relative Fatty Acid FAMEs Composition Composition Proposed Oil Identity 10. Discussion (Write 1-2 paragraphs including the following.) List the identity and adjusted area percent values of all FAMEs in the unknown sample. How do the calculated percent composition values compare to the expected values based on the relative fatty acid composition of common oils? What is the proposed identity of the unknown oil? In what order did the FAMEs elute from the GC column? Does the order of elution of the compounds make sense based on the size and unsaturation of the fatty acids and their corresponding FAMEs? Briefly explain. Include a short comment addressing what could be done differently to improve the experimental results, if repeated. Be sure to attach GC sample chromatogram!