Abiotic Factors Used in Measuring Water Quality Dissolved Oxygen
advertisement

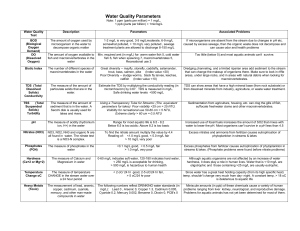
Abiotic Factors Used in Measuring Water Quality Dissolved Oxygen Dissolved oxygen (DO) is a measure of how much oxygen is dissolved in the water. DO is one of the best indicators of the health of an aquatic ecosystem. You can't tell by looking at water that there is oxygen in it. But, if you look at a closed bottle of soda, you don't see the carbon dioxide dissolved in that until you shake it up and open the top. The oxygen dissolved in aquatic ecosystems is crucial for the organisms living in it. As the amount of DO drops below normal levels in aquatic ecosystems, the water quality is harmed and organisms begin to die off. Although water molecules contain an oxygen atom, this oxygen is not what is needed by aquatic organisms. A small amount of oxygen molecules, measured in parts per million (ppm) of water, is actually dissolved in water. Most natural aquatic ecosystems require 5-6 ppm of DO to support a diverse population if life. Oxygen enters the water by direct absorption from the atmosphere or by plant photosynthesis. The oxygen is used by plants and animals for respiration and by bacteria which consume oxygen during the process of decomposition. When organic matter such as animal waste or improperly treated wastewater enters a body of water, algae growth increases and the DO levels decrease as the plant material dies off and is decomposed by bacteria that consume DO. A decrease in the DO levels is usually an indication of some type of pollutant, usually organic waste. Decreases in DO levels can cause changes in the types and numbers of aquatic macroinvertebrates which live in an aquatic ecosystem. Some species of macroinvertebrates are sensitive to decreased DO levels. As DO levels decrease, these pollution-sensitive macroinvertebrates are replaced by pollution-tolerant species that require lower DO levels for survival. DO levels change and vary in an aquatic ecosystem based on location (shallow fast moving water contains more dissolved oxygen), time of day, the weather, and temperature (the colder the water temperature the more DO that can exist in the water). Temperature The temperature of an aquatic ecosystem is of great importance because it can influence DO levels, the rate at which algae and aquatic plants carry out photosynthesis, and the metabolic rate of aquatic organisms. Since cold water can hold more DO than warm water, one of the problems we have created that affects water quality is thermal pollution. Thermal pollution is the introduction of warm water into an aquatic ecosystem. Sources include industries, power plants, and storm-drain runoff which has been warmed on streets, parking lots and sidewalks. In addition, human activities such as cutting down trees, the removal of vegetation around the water, and construction can lead to an increase in water temperature. These practices increase soil erosion which leads to an increase in dissolved solids in the water. As dissolved solids increase, the water becomes cloudy and absorbs more solar energy which increases the water temperature. An aquatic ecosystem that increases in water temperature holds less DO. Less DO affects animals that need DO for respiration. Increases in temperature also cause changes in aquatic plants. As the temperature increases, the rate of photosynthesis increases. As photosynthesis increases, the number of aquatic plants increase. This can lead to an increase in the number of plants that die and are decomposed by bacteria which consume DO in the process. Increases in temperature also increase the metabolic rate of organisms which live in aquatic ecosystems. As metabolic rate increases, the demand of DO increases and the rate at which the organisms go through their life cycles increases which would affect the food chain Biotic Factors Used in Measuring Water Quality Aquatic Macroinvertebrates Aquatic macroinvertebrates are found in lakes, streams, ponds, and marshes and help maintain the health of the aquatic ecosystem by eating bacteria and dead, decaying plants and animals. The following are the four primary groups of macroinvertebrates: Insects: stoneflies, mayflies, damselflies, dragonflies, beetles, caddisflies Crustaceans: crayfish, scuds, aquatic sow bugs Annelids: aquatic worms, flat worms, leeches Mollusks: clams, mussels, snails, limpets Overall water quality affects which types of organisms can survive in a body of water. Water quality may include the amounts of DO, pollutants and pH level. Some macroinvertebrates such as stoneflies, mayflies and water pennies require a high level of DO and their abundance is an indication of good water quality. Other macroinvertebrates can survive at a lower DO level because they can come to the surface to get oxygen or carry a bubble of air with them around their bodies. Several species of macroinvertebrates, like aquatic worms and leeches, can indicate an aquatic ecosystem with lower DO levels. Lower DO levels are often associated with polluted waters while higher levels indicate good water quality. Macroinvertebrates as Water Quality Indicators As pollutants enter an aquatic ecosystem, many macroinvertebrates will die because they are sensitive to changes in the ecosystem. Individual species of macroinvertebrates are sensitive to specific toxins, so the presence of certain types of insects gives clues to the types of pollutants present. In general, the greater variety of macroinvertebrates that live in a section of stream, the more stable, and healthy the environment. Pollution-free waters host a distinctive collection of pollutionsensitive macroinvertebrates, highly polluted water will host pollution-tolerant macroinvertebrates. A stream with different types of mayflies, stoneflies, and water pennies will be a much healthier environment than one that hosts only aquatic worms and leeches. Federal and state regulators have begun to rely on macroinvertebrate surveys as biological indicators to determine stream quality. This is because macroinvertebrates are sensitive to change; cannot easily escape changes in water quality; and they can be easily collected with very little expense. Chemical tests remain effective in cases of serious water pollution, where they can sometimes identify an exact toxin. But such testing can be an expensive process and the results may be inconclusive because pollutants quickly dilute and might only enter a waterway sporadically.