Word - ASDL Community
advertisement

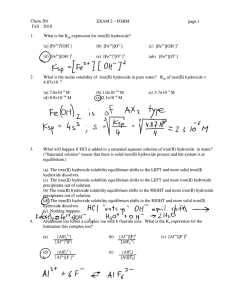
Lithia Water Analysis Module Solubility Equilibria Assessment Question Set #1 Q1. Write the balanced reaction for the dissolution of iron (III) hydroxide in water. Q2. Write the equilibrium constant expression for the solubility of iron (III) hydroxide. Q3. Calculate the molar solubility of iron (III) hydroxide in pure water. Q4. Calculate the molar solubility of iron (III) hydroxide at pH 6.4, and compare it to the calculated molar solubility of iron (III) hydroxide in Q3. Q5. Calculate the molar solubility of iron (III) hydroxide at pH 6.4 when the ionic strength of the solution is 0.10 mol L-1. Q6. To prevent precipitation of iron (III) hydroxide in household water systems, hydrogen peroxide is often added as an oxidizing agent to municipal water sources containing iron (II) ion concentrations above 0.3 mg L-1. Compare the effectiveness of this treatment at pH 6.4 versus pH 2.0 at a dissolved iron (i.e. Fe2+) concentration of 0.3 mg L-1.