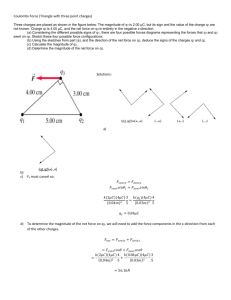
APPHY6.PS electrostatics SOLUTIONS
advertisement

APPHY6.PS electrostatics SOLUTIONS Chapters 16 and 17 P471 44. Set the magnitude of the electric force equal to the magnitude of the force of gravity and solve for the distance. Felectric = Fgravitational ® k r=e 45. 46. e2 r2 = mg ® k (8.988 ´ 109 N × m 2 /C2 ) = (1.602 ´ 10-19 C) = 5.08 m mg (9.11 ´ 10-31 kg)(9.80 m/s2 ) Water has an atomic mass of 18, so 1 mole of water molecules has a mass of 18 grams. Each water molecule contains 10 protons. æ 6.02 ´ 1023 H O molecules ö æ 10 protons ö æ 1.60 ´ 10-19 C ö 9 2 75 kg ç ÷ç ÷ = 4.0 ´ 10 C çè ÷ø è 1 molecule ÷ø çè 0.018 kg proton ø Calculate the total charge on all electrons in 3.0 g of copper and compare 32m C to that value. The molecular weight of copper is 63.5 g, and its atomic number is 29. æ 1 mole ö æ 6.02 ´ 1023 atoms ö æ 29 e ö æ 1.602 ´ 10-19 C ö 5 Total electron charge = 3.0 g ç ç ÷ç ÷ = 1.32 ´ 10 C ÷ø ç mole atoms 1 e è 63.5 g ÷ø è è ø è ø Fraction lost = 47. 32 ´ 10-6 C 1.32 ´ 10 C 5 = 2.4 ´ 10-10 Use Eq. 16–4a to calculate the magnitude of the electric charge on the Earth. We use the radius of the Earth as the distance. E=k Q r2 ® Q= Er 2 (150 N/C)(6.38 ´ 106 m)2 = = 6.8 ´ 105 C k 8.988 ´ 109 N × m 2 /C2 Since the electric field is pointing toward the Earth’s center, the charge must be negative. 48. (a) From Problem 47, we know that the electric field is pointed toward the Earth’s center. Thus an electron in such a field would experience an upward force of magnitude FE = eE. The force of gravity on the electron will be negligible compared to the electric force. FE = eE = ma ® a= (b) eE (1.602 ´ 10-19 C)(150 N/C) = = 2.638 ´ 1013 m/s2 » 2.6 ´ 1013 m/s2 , up -31 m (9.11 ´ 10 kg) A proton in the field would experience a downward force of magnitude FE = eE. The force of gravity on the proton will be negligible compared to the electric force. APPHY6.PS electrostatics SOLUTIONS Chapters 16 and 17 FE = eE = ma ® a= (c) 49. eE (1.602 ´ 10-19 C)(150 N/C) = = 1.439 ´ 1010 m/s2 » 1.4 ´ 1010 m/s2 , down m (1.67 ´ 10-27 kg) For the electron: a 2.638 ´ 1013 m/s2 = » 2.7 ´ 1012 2 g 9.80 m/s For the proton: a 1.439 ´ 1010 m/s2 = » 1.5 ´ 109 g 9.80 m/s2 For the droplet to remain stationary, the magnitude of the electric force on the droplet must be the same as the weight of the droplet. The mass of the droplet is found from its volume times the density of water. Let n be the number of excess electrons on the water droplet. FE = |q|E = mg ® neE = 43 p r 3r g ® n= 50. 4p r 3r g 4p (1.8 ´ 10-5 m)3 (1.00 ´ 103 kg/m 3 )(9.80 m/s2 ) = = 9.96 ´ 106 » 1.0 ´ 107 electrons -19 3eE 3(1.602 ´ 10 C)(150 N/C) There are four forces to calculate. Call the rightward direction the positive direction. The value of k is 8.988 ´ 109 N × m 2 /C2 and the value of e is 1.602 ´ 10-19 C. Fnet = FCH + FCN + FOH + FON = k(0.40e)(0.20e) é 1 1 1 1 ù + + êú -9 2 2 2 2 (1 ´ 10 m) êë (0.30) (0.40) (0.18) (0.28)2 ûú = 2.445 ´ 10-10 N » 2.4 ´ 10-10 N 51. The electric force must be a radial force in order for the electron to move in a circular orbit. FE = Fradial ® k rorbit = k 52. Q2 mu 2 Q2 2 rorbit = mu 2 rorbit ® = (8.988 ´ 109 N × m 2 /C2 ) (1.602 ´ 10-19 C)2 (9.109 ´ 10-31 kg)(2.2 ´ 106 m/s)2 = 5.2 ´ 10-11 m If all of the angles to the vertical (in both cases) are assumed to be small, then the spheres only have horizontal FT1 F 1 displacement, and the electric force of repulsion is always 2 T2 horizontal. Likewise, the small-angle condition leads to tanq » sinq » q for all small angles. See the free-body FE1 m g m2 g diagram for each sphere, showing the three forces of gravity, 1 tension, and the electrostatic force on each charge. Take the right to be the positive horizontal direction and up to be the positive vertical direction. Since the spheres are in equilibrium, the net force in each direction is zero. (a) å F1x = FT1 sinq1 - FE1 = 0 å F1y = FT1 cosq1 - m1g ® FE1 = FT1 sinq1 ® FT1 = m1g mg ® FE1 = 1 sinq1 = m1g tanq1 = m1gq1 cosq1 cosq1 FE2 APPHY6.PS electrostatics SOLUTIONS Chapters 16 and 17 A completely parallel analysis would give FE2 = m2 gq 2 . Since the electric forces are a Newton’s third law pair, they can be set equal to each other in magnitude. Note that the explicit form of Coulomb’s law was not necessary for this analysis. FE1 = FE2 ® m1gq1 = m2 gq2 ® q1 /q 2 = m2 /m1 = 1 (b) The horizontal distance from one sphere to the other, represented by d, is found by the small-angle approximation. See the diagram. Use the relationship derived above that FE = mgq to solve for the distance. d = (q1 + q 2 ) = 2 q1 ® q1 = m1gq1 = FE1 = kQ(2Q) d2 d 2 d = mg 2 ® æ 4 kQ 2 ö d =ç ÷ è mg ø 53.Because of the inverse square nature of the electric field, any location where the field is zero must be closer to the weaker charge (Q2 ). Also, in between the two l 1 2 l l sin 1 1/3 Q1 l sin 2 Q2 d l charges, the fields due to the two charges are in the same direction and cannot cancel. Thus the only place where the field can be zero is closer to the weaker charge, but not between them. In the diagram, this means that must be positive. Evaluate the net field at that location. E = -k Q2 2 +k Q2 = Q1 - Q2 Q1 ( + d) d= 2 =0 ® Q2 ( + d)2 = Q1 5.0 ´ 10-6 C 2.5 ´ 10-5 C - 5.0 ´ 10-6 C 2 ® (2.4 m) = 1.9 m from Q2 , 4.3 m from Q1 pp 498-499 68. (a) The energy is related to the charge and the potential difference by Eq. 17–3. DPE = qDV (b) ® DV = DPE 5.2 ´106 J = = 1.3´106 V q 4.0 C The energy (as heat energy) is used to raise the temperature of the water and boil it. Assume that room temperature is 20°C. Use Eq. 14–2 and Eq. 14–4. Q = mcDT + mLF ® m= 69. Q 5.2 ´106 J = = 2.0 kg æ cDT + LF æ J ö 5 J ö 4186 (80 C°) + 22.6 ´10 çè çè kg ×°C ÷ø kg ÷ø The electric force on the electron must be the same magnitude as the weight of the electron. The magnitude of the electric force is the charge on the electron times the magnitude of the electric field. The electric field is the potential difference per meter: E = V /d. FE = mg; FE = q E = eV /d ® eV /d = mg ® V= mgd (9.11´ 10-31 kg)(9.80 m/s2 )(0.024 m) = = 1.3´10-12 V e 1.60 ´10-19 C APPHY6.PS electrostatics SOLUTIONS Chapters 16 and 17 Since it takes such a tiny voltage to balance gravity, the thousands of volts in a television set are more than enough (by many orders of magnitude) to move electrons upward against the force of gravity. 70. The energy stored in the capacitor is given by Eq. 17–10, PE = 1 CV 2 . As the plates are separated, 2 the voltage is held constant by the battery. Since the separation distance has doubled, the capacitance has been multiplied by 1 2 , using Eq. 17–8. Thus the stored energy has been multiplied by ½. 71. The capacitor is charged and isolated, so the amount of charge on the capacitor cannot change. (a) The stored energy is given by Eq. 17–10, PE = 1 QV . The charge does not change, and the 2 potential difference is doubled, so the stored energy is multiplied by 2. (b) Q2 . The charge does not change. The C capacitance would be reduced by a factor of 2 if the plate separation were doubled, according to Eq. 17–8, so the stored energy is multiplied by 2. The stored energy can also be given by PE = 1 2 72.The energy in the capacitor, given by Eq. 17–10, is the heat energy absorbed by the water, given by Eq. 14–2. PE = Qheat ® 1 CV 2 2 = mcDT ® æ J ö 2(2.8 kg) ç 4186 (95°C - 21°C) kg ×°C ÷ø è 2mcDT V= = = 658 V » 660 V C 4.0 F 73. The proton would gain half the kinetic energy as compared to the alpha particle. The alpha particle has twice the charge of the proton, so it will have twice the potential energy for the same voltage. Thus the alpha will have twice the kinetic energy of the proton after acceleration. 74. We assume there is a uniform electric field between the capacitor plates, so that V = Ed. Combine Eq. 17–7 with Eq. 17–8 and Eq. 17–4. Q = CV = e0 A d V = e0 A V = e 0 AE = (8.85 ´ 10-12 C2 /N × m 2 )(65 ´ 10-4 m 2 )(3.0 ´ 106 V/m) d = 1.7 ´10-7 C 75. Use Eq. 17–5 to find the potential due to each charge. Since the triangle is equilateral, the 30-60-90 triangle relationship says that the distance from a corner to the midpoint of the opposite side is 3 /2. VA = k(-Q) k(-3Q) k(Q) 2kQ æ 1 ö kQ + + = -4 + = -6.85 ç ÷ /2 /2 è 3 /2 3ø VB = k(-Q) k(Q) k(-3Q) kQ kQ + + = -2 3 = -3.46 /2 /2 3 /2 VC = k(Q) k(-3Q) k(-Q) 2kQ æ 1 ö kQ + + =2+ = -5.15 ç ÷ /2 /2 è 3 /2 3ø B Q A Q C 3Q APPHY6.PS electrostatics SOLUTIONS Chapters 16 and 17 76. The energy is given by Eq. 17–10. Calculate the energy difference for the two different amounts of charge and then solve for the difference. Q PE = 1 2 Q= 77. (a) 2 ® DPE = C 1 2 (Q + DQ)2 1 Q 2 1 DQ -2 = [(Q + DQ)2 - Q 2 ] = [2Q + DQ] ® C C 2C 2C C(DPE) 1 (17.0 ´10-6 F)(15.2 J) 1 - 2 DQ = - 2 (13.0 ´10-3 C) = 13.4 ´10-2 C DQ (13.0 ´ 10-3 C) Because of the inverse square nature of the electric field, any location where the field is zero must be closer to the weaker charge (q2 ). Also, in between x d q2 0 q1 0 the two charges, the fields due to the two charges are parallel to each other (both to the left) and cannot cancel. Thus the only places where the field can be zero are closer to the weaker charge, but not between them. In the diagram, they are labeled x. E=k x= (b) q2 q1 - q2 q2 x 2 -k d= q1 (d + x) 2 =0 ® q2 (d + x)2 = q1x 2 ® 2.6 ´10-6 C 3.4 ´10-6 C - 2.6 ´10-6 C (2.5 cm) = 17.42 cm » 17 cm left of q2 The potential due to the positive charge is positive d x1 x2 everywhere, and the potential due to the negative charge is negative everywhere. Since the negative q2 0 q1 0 charge is smaller in magnitude than the positive charge, any point where the potential is zero must be closer to the negative charge. Consider locations between the charges (x1 ) and to the left of the negative charge (x2 ) as shown in the diagram. Vlocation 1 = kq1 kq q2 d (-2.6 ´10-6 C)(2.5 cm) + 2 = 0 ® x1 = = = 1.1 cm (d - x1 ) x1 (q2 - q1 ) (-6.0 ´10-6 C) Vlocation 2 = kq1 kq q2 d (-2.6 ´10-6 C)(2.5 cm) + 2 = 0 ® x2 = = = 8.1 cm (d + x2 ) x2 (q1 - q2 ) (0.8 ´10-6 C) So the two locations where the potential is zero are 1.1 cm from the negative charge toward the positive charge and 8.1 cm from the negative charge away from the positive charge.