AP Chemistry Equilibrium Homework
advertisement

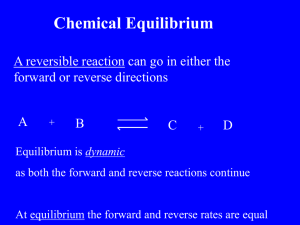
Name: AP Chemistry: Unit 14: Equilibrium: Homework Date: 1. Two colorless solutions are mixed in a stoppered flask. As the reaction proceeds, the resulting solution turns red, and a colorless gas is formed. After a few minutes, no more gas is evolved but the red color remains. What evidence is there that equilibrium has been established? 2. At a given temperature, analysis of an equilibrium mixture represent below is given as: SO2(g) + NO2(g) <=====> SO3(g) + NO(g) where [SO2] = 4.0 M, [NO2] = 0.50 M, [SO3] = 3.0 M, [NO] = 2.0 M Find the value of Keq. 3. For each of these reactions, indicate the property which might be observed in order to determine when equilibrium has been reached. a) PCl5(g) <====> PCl3(g) + Cl2(g) b) CaCO3(s) <=====> CaO(s) + CO2(g) c) H2O(l) <====> H2O(g) d) Cl2(g) + 2 H2(g) <====> H2(g) + 2 HCl(g) e) 2 HBr(g) <====> H2(g) + Br2(g) 4. Consider the reaction: N2O4(g) <=====> 2 NO2(g) Keq = 0.87 at 55oC ; This is an exothermic reaction. What is the effect of each of these changes upon the concentration of N2O4 at equilibrium? a) b) c) d) e) increasing the temperature. increasing the volume. adding more NO2(g) to the system without changing pressure or temperature. adding He gas to the container. adding a catalyst. 5. The reaction between nitrogen and oxygen to form NO(g) is represented by the chemical equation N2 (g) + O2 (g) 2NO (g) Equilibrium concentrations of the gases at 1500 K are 1.7x10-3 mol/L for O2 and 6.4x10-3 mol/L for N2 and 1.1x10-5 mol/L for NO. Calculate the value of Kc at 1500 K for these data. 6. A sealed tube initially contains 9.84x10-4 mol of H2 and 1.38x10-3 mol of I2. It is kept at 350C until the reaction H2 (g) + I2(g) 2HI (g) comes to equilibrium. At equilibrium 4.73x10-4 moles of I2 are present. Calculate the number of moles of H2 and HI at equilibrium and the equilibrium constant Kc for the reaction. 7. Antimony pentachloride decomposes in a gas phase reaction at high temperatures. SbCl5 SbCl3 (g) + Cl2 (g) At some temperature, an equilibrium mixture in a 5.00 L container is found to contain 6.91 g of SbCl5 16.45 g of SbCl3 and 5.11 g of Cl2 Evaluate Kc. If 10.0 grams of SbCl5 is placed in the 5.00 L container and allowed to reach equilibrium at the same temperature, what will be the equilibrium concentrations of all of the species? 8. In a study of the formation of HI from its elements, H2(g) + I2(g) 2HI(g) equal amounts of H2 and I2 were placed in a container, which was then sealed and heated. a. On one set of axes, sketch concentration vs. time curves for H2 and HI, and explain how Q changes as a function of time. b. Is the value of Q different if [I2] is plotted instead of [H2]? 9. Balance each reaction and write its reaction quotient, Qc: a. NO(g) + O 2 (g) N2O3(g) b. SF 6 (g) + SO 3 (g) SO2F2(g) c. SClF 5 (g) + H 2 (g) S 2 F 1 0 (g) + HCl(g) 10. Balance each of the following examples of heterogeneous equilibria and write its reaction quotient, Qc: a. Na 2 O 2 (s) + CO 2 (g) Na 2 CO 3 (s) + O 2 (g) b. H2O(l) H2O(g) c. NH 4 Cl(s) NH 3 (g) + HCl(g) 11. Calculate Ke for each of the following equilibria: a. CO(g) + Cl 2 (g) COC12 (g); K p = 3.9 x10 -2 at1000. K b. S 2 (g) + C(s) CS2(g); Kp = 28.5 at 500. K 12. In an experiment to study the formation of HI(g), H2(g) + I2(g) 2HI(g) H2(g) and I2(g) were placed in a sealed container at a certain temperature. At equilibrium, [H 2 ] = 6.50X10 -5 M, [I2 ] = 1.06 X 10 -3 M, and [HI] = 1.87 X 10 -3 M. Calculate Kc, for the reaction at this temperature. 13. Gaseous PC1 5 decomposes according to the reaction PCl5(g) PC13(g) + Cl2(g) In one experiment, 0.15 mol of PCl5(g) was introduced into a 2.0-L container. Construct the reaction table for this process. 14. Ammonium hydrogen sulfide decomposes according to t following reaction, for which K p 0.11 at 250°C: NH4HS(s) H2S(g) + NH3 (g) If 55.0 g of NH4HS(s) is placed in a sealed 5.0-L container, is the partial pressure of NH 3(g) at equilibrium? 15. Even at high T, the formation of nitric oxide is not favored. N2(g) + O2(g) 2NO(g) Kc = 4.10x 10 -4 at 2000°C What is [NO] when a mixture of 0.20 mol of N2(g) and 0.15 mol of O2(g) reach equilibrium in a 1.0-L container at 2000°C? 16. Consider this equilibrium system: CO(g) + Fe 3O4(s) CO2(g) + 3FeO(s) How does the equilibrium position shift as a result of each of the following disturbances? a. CO is added. b. CO2 is removed by adding solid NaOH. c. Additional Fe304(s) is added to the system. d. Dry ice is added at constant temperature. 17. Predict the effect of increasing the container volume on the amounts of each reactant and product in the following reactions: a. F2(g) 2F(g) b. 2CH4(g) C2H2(g) + 3H2(g) 18. Predict the effect of increasing the temperature on the amounts of products in the following reactions: a. CO(g) + 2H2(g) CH3OH(g) H= —90.7 kJ b. C(s) + H 2 O(g) CO(g) + H 2 (g) H= 131 kJ c. 2NO 2 (g) 2NO(g) + O 2 (g) (endothermic) d. 2C(s) + O 2 (g) 2CO(g) (exothermic) 19. The oxidation of SO2 is the key step in H2SO4 production: SO2(g) + ½ O2(g) SO3(g) H= -99.2 kJ a. What qualitative combination of T and P maximizes SO3 yield? b. How does addition of 02 affect Q? K? c. Why is catalysis used for this reaction? 20. One of the most important industrial sources of ethanol is reaction of steam with ethylene derived from crude oil: C2H4(g) + H2O(g) C2H5OH(g) Hr° = -47.8 kJ Kc, = 9 X 103 at 600. K a. At equilibrium, PC2H4 = 200 atm and PH2O = 400. atm. Calculate PC2H4. b. Is the highest yield of ethanol obtained at high or low pressures? High or low temperatures? c. In ammonia manufacture, the yield is increased by condensing the NH3 to a liquid and removing it from the vessel. Would condensing the C2H5OH have the same effect for ethanol production? Explain. 21. The following reaction is sometimes used to produce the H2 needed for the synthesis of ammonia: CH4(g) + CO2(g) 2CO(g) + 2H2(g) a. What is the percent yield of H2 when an equimolar mixture of CH4 and CO2 with a total pressure of 20.0 atm reaches equilibrium at 1200. K, at which Kp = 3.548 X 106? b. What is the percent yield of H2 for this system at 1300. K at which Kp = 2.626 X 107? 22. You are a member of a research team of chemists discussing the plans to operate an ammonia processing plant: N2(g) + 3H2(g) 2NH3(g) a. The plant operates at close to 700 K, at which Kp is 1.00X 10-4, and employs the stoichiometric 1/3 ratio of N2/H2. At equilibrium, the partial pressure of NH3 is 50. atm. Calculate the partial pressures of each reactant and Ptotal. b. One member of the team makes the following suggestion: since the partial pressure of H2 is cubed in the reaction quotient, the plant could produce the same amount of NH3 if the reactants were in a 1/6 ratio of N2/H2 and could do so at a lower pressure, which would cut operating costs. Calculate the partial pressure of each reactant and Ptotal under these conditions, assuming an unchanged partial pressure of 50. atm for NH3. Is the team member's argument valid? 23. The two most abundant atmospheric gases react to a tiny extent at 298 K in the presence of a catalyst: N2(g) + O2(g) 2NO(g) K p = 4.35X10 -3 1 a. What are the equilibrium pressures of the three components when the atmospheric partial pressures of O2 (0.210 atm) and of N2 (0.780 atm) are put into an evacuated 1.00-L flask at 298 K with catalyst? b. What is Ptotal in the container? c. Find Kc for this reaction at 298 K. 24. Using your knowledge of the Brønsted-Lowry theory of acids and bases, write equations for the following acid-base reactions and indicate each conjugate acid-base pair: a) HNO3 + OH- b) CH3NH2 + H2O c) OH- + HPO4-2 25. The compound NaOH is a base by all three of the theories we discussed in class. However, each of the three theories describes what a base is in different terms. Use your knowledge of these three theories to describe NaOH as an Arrhenius base, a Brønsted-Lowry base, and a Lewis base. 26. When hydrogen chloride reacts with ammonia, ammonium chloride is formed. Write the equation for this process, and indicate which of the reagents is the Lewis acid and which is the Lewis base. 27. Write an equation for the reaction of potassium metal with hydrochloric acid. 28. Borane (BH3) is a basic compound, but doesn’t conduct electricity when you dissolve it in water. Explain this, based on the definitions of acids and bases that we discussed in class. 29. What is the hydroxide concentration in a solution which results from pouring 100 mL of 0.010 M HCl together with 200 mL of 0.030M Ca(OH)2(aq) solution? Assume that the liquid volumes are additive. a) 0.020 M b) 0.0183 M c) 0.0367 M d) 0.060 M e) 0.030 M 30. What is the molarity of 1.0 N HI? a) 7.8 x 10-3 b) 128 c) 1.0 d) 2.0 31. What is the hydronium ion concentration in a solution which is 0.10 M HNO3(aq)? a) 0.30 M b) 0.10 M c) 6.7 x 10-3 M d) 1.0 x 10-7 M e) 2.1 x 10-2 M 32. What is the hydroxide ion concentration in 1.0 M HBr? a) 1.0 M b) 1.0 x 10-13 M c) 1.0 x 10-14 M d) 1.0 x 10-7 M 33. What is the pH of 1.0 x 10-3 M aqueous HClO4? a) 10-7 b) -3.0 c) 0.0 d) 3.0 e) 1.0 34. What is the hydronium ion concentration in a solution which has [OH-] = 1.0 x 10- 2 M? a) 1.0 x 10-12 M b) 1.0 x 10-2 M c) 1.0 x 10-16 M d) 12 e) 1.0 x 10-7 M 35. Identify the INCORRECT statement: a) As the pH increases the hydroxide ion decreases. b) As the pH increases the hydronium ion concentration decreases. c) As the pH increases the Kw of water remains the same. d) As the pH increases the product [H3O+][OH-] remains constant. e) As the pH increases the solution becomes less acidic and more alkaline. 36. Identify the most INCORRECT statement: a) Blood has a pH near 7. b) Stomach juices have a pH near 2. c) Vinegar has a pH near 10. d) Glass cleaner has a pH near 12. e) Drano has a pH near 14. 37. Which acid is the weakest acid? Refer to the table of acids given. a) HCN b) 3 HNO2 c) HAc d) HOCl e) HF 38. What is the Ka of HNO2(aq)? Refer to the table of acids given. a) -0.525 b) 3.35 c) 0.525 d) 4.5 x 10-4 e) 1.8 x 10-5 39. What is the pH of a 0.10 M solution of HF in water? The Ka = 7.2 x 10- 4. a) -2.07 b) 8.5 x 10-3 c) 0.10 d) 3.14 e) 2.1 40. What is the percent ionization of the acid in Problem #16? a) 100% b) 8.5% c) 0.11% d) 0.0011% e) 0.08% 41. What is the pH of a 0.20 M aqueous solution of the base methylamine, CH3NH2? Kb = 5.0 x 10-4. a) 2.0 b) 12.0 c) 16.0 d) 10.0 e) 0.01 42. For each of the following pairs of molecules, the red molecule is the stronger acid based on molecular structure and the stability of the conjugate base. Explain the reasoning. (a) HC CH H2C CH2 (b) O O O (c) NH3 PH3 43. Arrange the following in order of increasing acidity (weakest acid first). I) H2O (a) (b) (c) (d) (e) II) H3O+ III) HCO2H I < II < III I < III < II III < II < I III < I < II II < III < I 44. What role does CH3CH=CH2 play in this reaction? CH3CH=CH2 + HCl (a) (b) (c) (d) (e) (f) Brønsted-Lowry acid Lewis acid Brønsted-Lowry base Lewis base both (a) and (b) both (c) and (d) CH3CHCH3 + Cl 45. Explain the observed trend in acid strength for the following five compounds: O O O I Br OH pKa = 4.76 O Cl OH pKa = 3.15 O F OH pKa = 2.86 ACID pH Ka -------------------------------HF 3.14 7.2 x 10-4 HNO2 3.35 -------HAc 4.74 1.8 x 10-5 HOC 7.45 3.5 x 10-8 HCN 9.4 4.0 x 10-10 BASE pH pOH Kb ----------------------------------------------------------------ammonia 9.26 4.74 1.8 x 10-5 methylamine 10.7 3.30 5.0 x 10-4 dimethylamine 10.87 3.13 7.4 x 10-4 trimethylamine 9.87 4.13 7.4 x 10-5 pyridine 5.18 8.82 1.5 x 10-9 46. Which of the following are Arrhenius acids? a. H2O b. Ca(OH)2 c. H3P03 d. HI 47. Write the Ka expression for each of the following in water: a. HNO2 b. CH3COOH c. HBrO2 48. Classify each as a strong or weak acid or base: a. H3AsO4 b. Sr(OH)2 c. HIO d. HClO4 OH pKa = 2.81 OH pKa = 2.66 49. Which solution has the higher pH? Explain. a. A 0.1 M solution of an acid with Ka = 1 X 10-4 or one with Ka = 4X10-5 b. A 0.1 M solution of an acid with pKa = 3.0 or one with pKa = 3.5 c. A 0.1 M solution of a weak acid or a 0.01 M solution of the same acid d. A 0.1 M solution of a weak acid or a 0.1 M solution of a strong acid e. A 0.1 M solution of an acid or a 0.01 M solution of a base f. A solution of pOH 6.0 or one of pOH 8.0 50. How many moles of H30+ or OH- must you add to 5.6 L of HA solution to adjust its pH from 4.52 to 5.25? Assume a negligible volume change. 51. Like any equilibrium constant, KW changes with temperature. a. Given that autoionization is an endothermic process, does Kw, increase or decrease with rising temperature? Explain with an equation that includes heat as reactant or product. b. In many medical applications, the value of Kw at 37°C (body temperature) may be more appropriate than the value at 25°C, 1.0X 10-14. The pH of pure water at 37°C is 6.80. Calculate Kw, pOH, and [OH-] at this temperature. 52. In each equation, label the acids, bases, and congugate acid-base pairs: a. NH3 + H3PO4 NH4+ + H2PO4 b. CH 3 O - + NH 3 CH3OH + NH2c. HPO 4 2+ + HSO 4 H2PO4 + SO42- 53. In each of the following cases, would you expect the concentration of acid before and after dissociation to be nearly the same or very different? Explain your reasoning. a. A concentrated solution of a strong acid b. A concentrated solution of a weak acid c. A dilute solution of a weak acid d. A dilute solution of a strong acid 54. A 0.15 M solution of butanoic acid, CH3CH2CH2COOH, contains 1.51 X10-3 M H30+. What is the Ka of butanoic acid? 55. Nitrous acid, HNO2, has a Ka of 7.1 X10-4. What are [H3 O+ ], [NO2 -], and [OH -] in 0.60 M HNO 2? 56. The weak acid HZ has a Ka of 2.55 X 10-4. a. Calculate the pH of 0.075 M HZ. b. Calculate the pOH of 0.045 M HZ. 57. Write balanced equations and Kb expressions for these Bronsted-Lowry bases in water: (a) Pyridine, C5H5N (b) CO32- 58. Choose the stronger acid in each of the following pairs: a. H2Se or H3As b. B(OH)3 or Al(OH)3 c. HBrO2 or HBrO 59. What determines whether an aqueous solution of a salt will be acidic, basic, or neutral? Give an example of each type of salt. 60. Why is aqueous NaF basic but aqueous NaCl neutral? 61. Explain with equations and calculations, when necessary, whether an aqueous solution of each of these salts is acidic, basic, or neutral: a. KBr b. NH4I c. KCN 62. How do Lewis acids differ from Bronsted-Lowry acids? How are they similar? Do Lewis bases differ from Bronsted-Lowry bases? Explain. 63. Identify the Lewis acid and Lewis base in each equation: a. Na+ + 6H2O Na(H2O)6+ b. CO2 + H2O H2CO3 c. F- + BF3 BF464. Classify the following as Arrhenius, Bronsted-Lowry, or Lewis acid-base reactions. A reaction may fit all, two, one, or none of the categories: a. Ag + + 2NH3 Ag(NH3)2+ b. H 2 SO 4 + NH 3 HSO 4 - + NH 4 + c. 2HCl H2 + Cl2 d. AlCl3 + Cl- AlC1465. Hemoglobin (Hb) transports oxygen in the blood: HbH+(aq) + O2(aq) + H2O(l) HbO2(aq) + H3O+(aq) In blood, [H3O+] is held nearly constant at 4 X10-8 M. a. How does the equilibrium position change in the lungs? b. How does it change in O2-deficient cells? c. Excessive vomiting may lead to metabolic alkalosis, in which [H3O+] in blood decreases. How does this condition affect the ability of Hb to transport O2? d. Diabetes mellitus may lead to metabolic acidosis, in which [H3O+] in blood increases. How does this condition affect the ability of Hb to transport O2? 66. In standardizing an HCl solution of unknown concentration, 21.50 mL of HCl solution are found to reach the endpoint in a titration with 4.086 g of Na2CO3. (MW = 106.0 g/mol) What is the molarity of the HCl solution? a) 1.79 M b) 3.58 M c) 0.896 M d) 3.6 x 10-3 M 67. What volume of 3.0 N H3PO4 would exactly neutralize 250.0 mL of aqueous 2.0 N NaOH? a) 375 mL b) 166.7 mL c) 250.0 mL d) 45 mL 68. What reagent would be the best choice for a primary standard to standardize a solution of KOH of unknown concentration? a) KHP (potassium hydrogen phthalate) b) sodium carbonate c) sodium hydroxide d) HCl e) KCl 69. What volume of 0.050 M NaOH(aq) will exactly neutralize 100.0 mL of 0.075 M HBr solution? a) 100.0 mL b) 7.5 mL c) 6.6 x 10-3 mL d) 150 mL e) 120 mL 70. What volume of 0.200 M H2SO4(aq) would exactly neutralize 2.00 L of 1.0 x 10-3 M Al(OH)3? a) 6.7 mL b) 10 mL c) 15 mL d) 1.00 L e) 75 mL 71. A buffer solution is prepared by the addition of 20.0 grams of HF and 21.0 grams of NaF to enough water to make 1.000 L of solution. The Ka of HF is 7.2 x 10-4. What is the pH of this solution? a) 3.44 b) 3.14 c) 2.84 d) 7.0 e)2.4 72. Which of the following pairs of compounds dissolved together in aqueous solution would NOT make a good buffer solution? a) HNO3/NaNO3 b) H3PO4/NaH2PO4 c) H2CO3/KHCO3 d) HCN/KCN 73. A buffer solution is 0.10 M HAc (acetic acid) and 0.10 M NaAc (sodium acetate) and we have 1.000 Liter of it. What is the pH after 0.010 moles of HCl are added to this solution? a) -2.0 b) 4.74 c) 4.82 d) 2.0 e) 4.65 74. We desire to make a buffer solution with a pH of 9.2. Which combination of compounds would be the best to use? Refer to the table of bases given. a) HAc/NaAc b) NH3/NH4Cl c) HCl/NaCl d) pyridine/pyridine chloride 75. A solution is prepared which is 1.0 M acetic acid. What would happen to the pH of this solution if potassium acetate were added to the solution? a) pH would go up b) pH would go down c) would not affect the pH 76. A buffer solution has a pH = 5.5. What is the [H3O+] concentration? a) 5.5 M b) 3.16 x 10-6 M c) 3.2 x 105 M d) 0.74 M e) 3.2 x 10-3 M 77. When a small amount of H3O+ is added to a buffer, does the pH remain constant? Explain. 78. What are the [H3O+] and the pH of a propanoic acid/propanoate buffer that consists of 0.35 M CH3CH2COONa and 0.15 M CH3CH2COOH (Ka of propanoic acid = 1.3 X 10-5)? 79. Find the pH of a buffer that consists of 1.3 M sodium phenolate (C6H5ONa) and 1.2 M phenol (C6H5OH) (pKa of phenol = 10.00). 80. Find the pH of a buffer that consists of 0.25 M NH3 and 0.15 M NH4C1 (pKb of NH3 = 4.75). 81. A buffer containing 0.2000 M of acid, HA, and 0.1500 M of its conjugate base, A-, has a pH of 3.35. What is the pH after 0.0015 mol of NaOH is added to 0.5000 L of this solution? 82. What is the difference between the end point of a titration and the equivalence point? Is the equivalence point always reached first? Explain. 83. The indicator cresol red has Ka 3.5 X10-9. Over what approximate pH range does it change color? 84. Calculate the pH during the titration of 40.00 mL of 0.1000 M HCl with 0.1000 M NaOH solution after the following additions of base: a. 0 mL b. 25.00 mL c. 39.00 mL d. 39.90 mL e. 40.00 mL f. 40.10 mL g. 50.00 mL. 85. Find the pH during the titration of 20.00 mL of 0.1000 M butanoic acid, CH3CH2CH2COOH (Ka = 1.54 X 10-5), with 0.1000 M NaOH solution after the following additions of titrant: a. 0 mL b. 10.00 mL c. 15.00 mL d. 19.00 mL e. 19.95 mL f. 20.00 mL g. 20.05 mL h. 25.00 mL 86. Why does pH affect the solubility of BaF2 but not of BaC12? 87. The solubility of silver carbonate is 0.032 M at 20°C. Calculate its Ksp. 88. The solubility of silver dichromate 8.3 X10-3 g/100 mL solution. Calculate its Ksp. 89. Find the molar solubility of SrCO3 (Ksp = 5.4X 10-10) in (a) pure water and (b) 0.13 M Sr(NO3)2? 90. Which compound in each pair is more soluble in water? a. Magnesium hydroxide or nickel(II) hydroxide b. Lead(II) sulfide or copper(II) sulfide c. Silver sulfate or magnesium fluoride 91. Does any solid Cu(OH)2 form when 0.075 g of KOH is dissolved in 1.0 L of 1.0X 10-3 M Cu(NO3)2? 92. Consider the dissolution of PbS in water: PbS(s) + H2O(l) Pb2+(aq) + HS- (aq) + OH-(aq) Adding aqueous NaOH causes more PbS to dissolve. Does this violate Le Chatelier's principle? Explain. 93. Write a balanced equation for the reaction of Ag(H2O)2+ in aqueous Na2S2O3. 94. Gout is caused by an error in nucleic acid metabolism that leads to a buildup of uric acid in body fluids, which is deposited as slightly soluble sodium urate (C5H3N4O3Na) in the soft tissues of joints. If the extracellular [Na] is 0.15 M and the solubility in water of sodium urate is 0.085 g/100. mL, what is the minimum urate ion concentration (abbreviated [Ur-]) that will cause a deposit of sodium urate? 95. The solubility of KCl is 3.7 Mat 20°C. Two beakers contain 100. mL of saturated KCl solution: 100. mL of 6.0 M HCl is added to the first beaker and 100. mL of 12 M HCl to the second. a. Find the ion-product constant of KCl at 20°C. b. What mass, if any, of KCl will precipitate from each beaker? 96. The normal pH of blood is 7.40 ± 0.05 and is controlled in part by the H2CO3/HCO3- buffer system. a. Assuming that the Ka value for carbonic acid at 25°C applies to blood, what is the [H2CO3]/[HCO3-] ratio in normal blood? b. In a condition called acidosis, the blood is too acidic. What is the [H2CO3]/[HCO3-] ratio in a patient whose blood pH is 7.20 (severe acidosis)?