N&V - UBC Blogs
advertisement

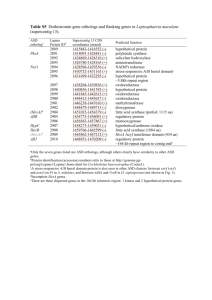
3-Minute N&V Write-Up Brianna Davies 29520111 Recently, there has been a big push to identify the genetic contributions to Autism Spectrum Disorder (ASD). Many ASD-risk genes have been identified through GWAS studies and 9 different genes in particular have been classified as highly associated with ASD. More recently, researchers have discovered that many of the ASD risk genes are co-expressed in networks during fetal neural development. This has prompted researchers to consider the idea that a gene regulation mechanism is at play. Further, it has been suggested that one ASD-risk gene may be responsible for regulating other associated ASD-risk genes and therefore contributing to co-expression networks identified. In this study, researchers chose to look at the potential regulatory role of the CHD8 gene, a previously identified ASD risk gene. The CHD8 gene codes for an ATPdependent chromatin remodeler, and 11 different de novo mutations in this gene have been identified in ASD patients. Further, it is known that CHD8 expression increases in neural tissue during development, making it a prime candidate for playing a regulatory role. This paper then set out to answer the following question: “Does CHD8 target other ASD genes during neurodevelopment?” Further, do gene knockdowns of CHD8 lead to unorganized regulation of ASD risk genes? To answer this question, researchers used CHiP-Seq technology to identify CHD8 binding sites on the genome of both human neural stem cells as well fetal brain tissue. Many genes were bound by CHD8 in both samples, identifying a group of genes expressed during both contexts. They found that approximately 90% of the CHD8 binding sites were at promoter regions. Further, CHD8 bound to the promoters of 47 previously identified ASD-risk genes found in both human neural stem cells and fetal brain tissue. Figure 1B on the slide shows the frequency of CHD8 binding to the promoters of ASD-risk genes previously identified, with that list being shown in Fig 1C. The researchers then went on to examine whether CHD8 binding was associated with histone modifications associated with active chromatin states. They found that 99% of the promoters bound by CHD8 in hNSCs were enriched with active chromatin markers, such as H3K27ac. As seen in Figure 1D, CHiP –Seq signal tracks for H3K27 and CHD8 are both shown to peak around the transcription start site of an ASD-gene (POGZ). This evidence supports the idea that CHD8 is found at the promoters of actively transcribed genes during fetal neurodevelopment, which are known to play a role in ASD risk. References: Cotney, J. et al. The autism-associated chromatin modifier CHD8 regulates other autism risk genes during human neurodevelopment. Nature Communications 6, 6404 (2015).