name: Hiep Ton
advertisement
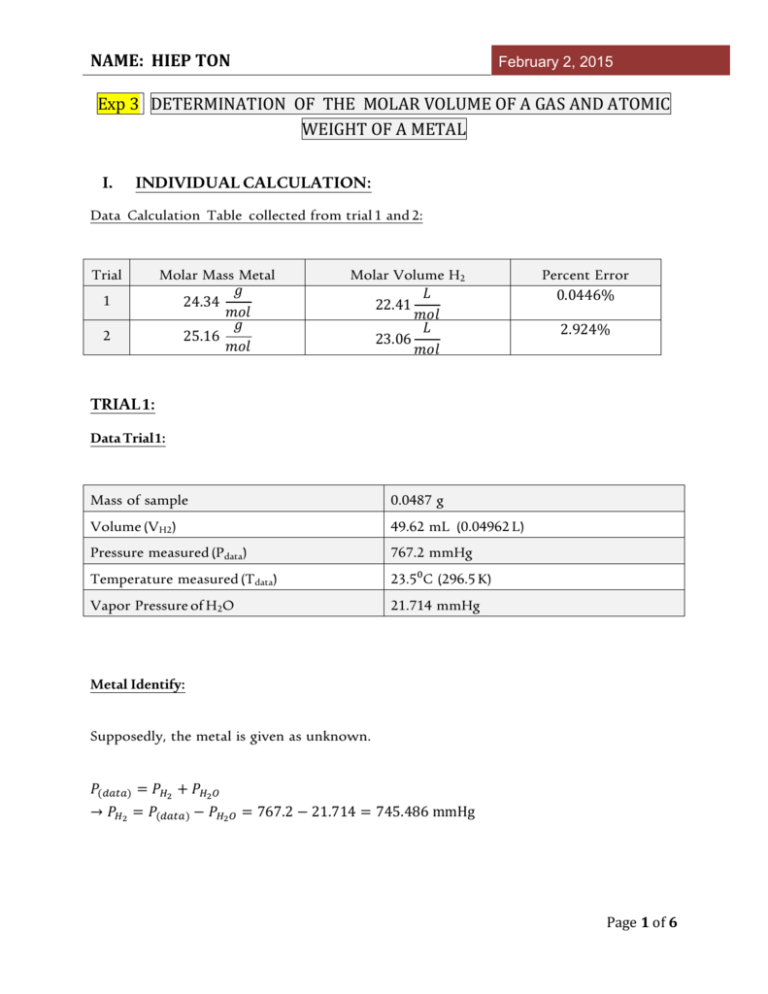
NAME: HIEP TON February 2, 2015 Exp 3 DETERMINATION OF THE MOLAR VOLUME OF A GAS AND ATOMIC WEIGHT OF A METAL I. INDIVIDUAL CALCULATION: Data Calculation Table collected from trial 1 and 2: Trial 1 2 Molar Mass Metal 𝑔 24.34 𝑚𝑜𝑙 𝑔 25.16 𝑚𝑜𝑙 Molar Volume H2 𝐿 22.41 𝑚𝑜𝑙 𝐿 23.06 𝑚𝑜𝑙 Percent Error 0.0446% 2.924% TRIAL 1: Data Trial 1: Mass of sample 0.0487 g Volume (VH2) 49.62 mL (0.04962 L) Pressure measured (Pdata) 767.2 mmHg Temperature measured (Tdata) 23.5⁰C (296.5 K) Vapor Pressure of H2O 21.714 mmHg Metal Identify: Supposedly, the metal is given as unknown. 𝑃(𝑑𝑎𝑡𝑎) = 𝑃𝐻2 + 𝑃𝐻2 𝑂 → 𝑃𝐻2 = 𝑃(𝑑𝑎𝑡𝑎) − 𝑃𝐻2 𝑂 = 767.2 − 21.714 = 745.486 mmHg Page 1 of 6 NAME: HIEP TON February 2, 2015 𝑃𝐻2 𝑉𝐻2 = 𝑃(𝑑𝑎𝑡𝑎) 𝑉2 → 𝑉2 = 𝑃𝐻2 𝑉𝐻2 745.486 𝑚𝑚𝐻𝑔 × 0.04962 𝐿 = = 0.048215609 𝐿 𝑃(𝑑𝑎𝑡𝑎) 767.2 𝑚𝑚𝐻𝑔 𝑃𝐻2 𝑉𝐻2 = 𝑛𝐻2 𝑅 𝑇(𝑑𝑎𝑡𝑎) → 𝑛𝐻2 745.486 ( 760 atm) × (0.048215609 𝐿) 𝑃𝐻2 𝑉𝐻2 = = = 2.000688 × 10−3 mol 𝑎𝑡𝑚. 𝐿 𝑅 𝑇(𝑑𝑎𝑡𝑎) (0.08206 ) × (296.5 K) 𝑚𝑜𝑙. 𝐾 𝑀 + 2𝐻𝐶𝑙 → 𝑀𝐶𝑙2 + 𝐻2 → 𝑚𝑜𝑙 𝑀 = 𝑚𝑜𝑙 𝐻2 → 𝑀 = 𝑚 0.0487 g 𝑔 = ≈ 24.34 −3 𝑛 2.000688 × 10 mol 𝑚𝑜𝑙 Metal Identity : Magnesium (Mg) Molar Volume Identify: Volume of H2: 𝑃(𝑆𝑇𝑃) 𝑉𝐻2 𝑃𝐻2 𝑉(𝑑𝑎𝑡𝑎) = 𝑇(𝑆𝑇𝑃) 𝑇(𝑑𝑎𝑡𝑎) → 𝑉𝐻2 = 𝑃𝐻2 𝑉(𝑑𝑎𝑡𝑎) 𝑇(𝑆𝑇𝑃) (745.486 mmHg) × (0.04962 L) × (273 𝐾) = = 0.044839335 L (760 mmHg ) × (296.5 𝐾) 𝑃(𝑆𝑇𝑃) 𝑇(𝑑𝑎𝑡𝑎) Page 2 of 6 NAME: HIEP TON February 2, 2015 Mole of H2: 𝑀 + 2𝐻𝐶𝑙 → 𝑀𝐶𝑙2 + 𝐻2 → 𝑚𝑜𝑙 𝐻2 = 𝑚𝑜𝑙 𝑀 = 2.000688 × 10−3 mo𝑙 Molar Volume of H2: → 𝑉 0.044839335 L 𝐿 = = 22.41195779 −3 𝑛 2.000688 × 10 mo𝑙 𝑚𝑜𝑙 Percent Error: % 𝐸𝑟𝑟𝑜𝑟 = (22.41195779 − 22.4) = 0.0446% 22.4 TRIAL 2: Data Trial 2: Mass of sample 0.0415 g Volume (VH2) 42.10 mL (0.04210 L) Pressure measured (Pdata) 767.2 mmHg Temperature measured (Tdata) 23.5⁰C (296.5 K) Vapor Pressure of H2O 21.714 mmHg Metal Identify: Supposedly, the metal is given as unknown. Page 3 of 6 NAME: HIEP TON February 2, 2015 𝑃(𝑑𝑎𝑡𝑎) = 𝑃𝐻2 + 𝑃𝐻2 𝑂 → 𝑃𝐻2 = 𝑃(𝑑𝑎𝑡𝑎) − 𝑃𝐻2 𝑂 = 767.2 − 21.714 = 745.486 mmHg 𝑃𝐻2 𝑉𝐻2 = 𝑃(𝑑𝑎𝑡𝑎) 𝑉2 → 𝑉2 = 𝑃𝐻2 𝑉𝐻2 745.486 𝑚𝑚𝐻𝑔 × 0.04210 L = = 0.040908447 𝐿 𝑃(𝑑𝑎𝑡𝑎) 767.2 𝑚𝑚𝐻𝑔 𝑃𝐻2 𝑉𝐻2 = 𝑛𝐻2 𝑅 𝑇(𝑑𝑎𝑡𝑎) → 𝑛𝐻2 745.486 ( 760 atm) × (0.040908447 𝐿) 𝑃𝐻2 𝑉𝐻2 = = = 1.649235529 × 10−3 mol 𝑎𝑡𝑚. 𝐿 𝑅 𝑇(𝑑𝑎𝑡𝑎) (0.08206 ) × (296.5 K) 𝑚𝑜𝑙. 𝐾 𝑀 + 2𝐻𝐶𝑙 → 𝑀𝐶𝑙2 + 𝐻2 → 𝑚𝑜𝑙 𝑀 = 𝑚𝑜𝑙 𝐻2 → 𝑀 = 𝑚 0.0415 g 𝑔 = ≈ 25.16317 𝑛 1.649235529 × 10−3 mol 𝑚𝑜𝑙 Metal Identity: Magnesium (Mg) Molar Volume Identify: Volume of H2: 𝑃(𝑆𝑇𝑃) 𝑉𝐻2 𝑃𝐻2 𝑉(𝑑𝑎𝑡𝑎) = 𝑇(𝑆𝑇𝑃) 𝑇(𝑑𝑎𝑡𝑎) Page 4 of 6 NAME: HIEP TON → 𝑉𝐻2 = February 2, 2015 𝑃𝐻2 𝑉(𝑑𝑎𝑡𝑎) 𝑇(𝑆𝑇𝑃) (745.486 mmHg) × (0.04210 L) × (273 𝐾) = = 0.038022961 L (760 mmHg ) × (296.5 𝐾) 𝑃(𝑆𝑇𝑃) 𝑇(𝑑𝑎𝑡𝑎) Mole of H2: 𝑀 + 2𝐻𝐶𝑙 → 𝑀𝐶𝑙2 + 𝐻2 → 𝑚𝑜𝑙 𝐻2 = 𝑚𝑜𝑙 𝑀 = 1.649235529 × 10−3 mol Molar Volume of H2: → 𝑉 0.038022961 L 𝐿 = = 23.0549 −3 𝑛 1.649235529 × 10 mol 𝑚𝑜𝑙 Percent Error: % 𝐸𝑟𝑟𝑜𝑟 = II. (23.0549 − 22.4) = 2.9236607% 22.4 POSTLAB QUESTIONS 1. If the metal strip had not been cleaned and an oxide film was left on the ribbon. Would the volume of hydrogen gas generated be higher or lower than the true value? Explain. Answer: If the oxide film was still on the ribbon, the volume of hydrogen gas generated would be lower than the true value. It is because the O2 would try to get the H2 and form water . Therefore, the amount of H2 would be lower Page 5 of 6 NAME: HIEP TON February 2, 2015 2. If some air enters the eudiometer tube, what effect will it have on the atomic weight determination? Answer: The air will increase the mole. Then, the molar mass of the metal will be decreased. Therefore, the metal cannot be determined correctly deal with the incorrect lower molar mass from the data calculation. 3. What is the value of R in SI units? Answer: III. 𝐽 𝑘𝑔 . 𝐾 PEERS EVALUATION Thi Bui A Very active, answer all the concerns and well communicated. Take responsibility for the task assigned and finish early. Really involved into group work. Tuyet Mai Nguyen A Not communicate well. Finish the task assigned and take care of unfinished part early. Really involved into group work. Jiale Yu A Finish the task assign and very helpful on calculation. Take responsible to finish any unfinished part. Really involved into group work. John Schubert F Not active. Not finish anything. Page 6 of 6









