H2 antagonists Histamine Imidazole ring Tele N and pi N
advertisement

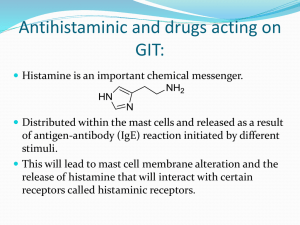
H2 antagonists Histamine - Imidazole ring Tele N and pi N - N pi is closest to the alkyl amine side chain and N tele is furthest away Ethyl amine side chain Tautomerism - Going from lowest pH – for example in the stomach To higher pH blood at pH7.4 – major form at pH 7.4 In the Stomach of the gastric parietal cell, the major form is the dication Histamine tautomerisation - Important for H1-receptor binding Gastric Parietal Cells - H2 antagonists inhibit gastric acid secretion elicited by histamine in a manner that is dosedependent and competitive. They also inhibit gastric acid secretion elicited by gastrin and, to a lesser extent, by ACh H2 antagonists inhibit basal, nocturnal and (to some extent) foodstimulated acid secretion. - The use of H2 antagonists in the treatment of gastroesophageal reflux disease (GERD) is feasible, but limited by a relatively short duration of action, and incomplete inhibition of food-stimulated gastric acid secretion. H2 antagonists also decrease secretion of intrisic factor, but not to the extent that will affect vitamin B12 absorption. H2 antagonists: Cimetidine - - Histamine was known to stimulate gastric acid production in vitro, however all known antihistamines of this time failed to block this action of histamine SKF researches proposed that there may be 2 types of histamine receptors and that suitably designed antagonists would be able to discriminate between them and inhibit gastric acid secretion. Early 1960’s in the search for a cure for ulcers, research team at SKF decided to look for an antihistamine that would block the secretion of gastric acid as a treatment for ulcers. Discovered in 1972 by Black et al Gastric parietal cells, guinea pig atria, uterus Control release of gastric acid from gastric parietal cells H2 agonists - Title of the slide should say H2 Agonists not H2 Antagonists No known receptor, just a postulated receptor, only compound that was known to interact with the proposed receptor was histamine itself, carried out structure activity studies using - analogues of histamine to find out if they caused the same physiological effects as histamine, ie release of gastric acid. From this they found that the structural requirements for H1 and H2 activity were slightly different H1 – does not require imidazole ring Design of an antagonist - After screening 200 compounds Having obtained some info about the requirements for H2 receptors, now need to design a molecule that would bind to a receptor but not activate it, to do this you need to alter the way that the molecule binds to the receptor. Guanylhistamine - - A partial agonist with very weak antagonist activity, ie it activates the H2-receptor, but not as much as histamine, if it is bound to the receptor it prevents histamine from binding therefore acting as antagonist. Guanidino group is basic and is therefore protonated at pH 7.4, so that it has a positive charge similar to histamine, but positive charge is delocalised through the three planar nitrogens. The positive charge can also be further away from the imidazole group meaning that the +ve charge may be interacting with a site on the receptor that histamine can’t reach Guanidine- Resonance Rules - - - Thinking an anchoring cation was essential in the structure of H2 antagonists, medicinal chemists first tried the guanidine group for R3, but it was not effective due to its excessive basicity. It has a pKa of 13 and, and even at pH 7.4, exists almost exclusively as its cationic conjugate acid. So why is guanidine such a strong base? The answer is again …Resonance! Check out the three guanidinium resonance forms that stabilize (weaken) the conjugate acid form of this strong base. Remember….the weaker one species is, the stronger its conjugate will be. Since this acid form is extremely weak due to extensive resonance stabilization, the conjugate base form (guanidine free base) will be strong, and will readily accept proton to form the stable conjugate acid. Therefore, the cationic acid form will predominate at physiologic pH. In fact, Henderson and Hasselbalch tell us that, at pH 7.4, the i/u ratio would be a whopping 106/1 (or close to it). Just for fun, calculate what the i/u ratio of this functional group would be at pH 2 (i.e., close to gastric pH 1.5). Extended analogues - replaced NH sith S, so that +ve charge is only delocalised through terminal 2 nitrogens, less likely to interact with “agonist binding site” - it was found to be a stronger antagonist, but still showed partial agonist activity. Other compounds in which one of the terminal NH2 groups wes replaced by methyl or methylthio groups showed that terminal amino grps were required for antagonist activity – propsed that charged guanidino group was interacting with a charged carboxylate group on receptor protein Agonist vs Antagonist - - Proposed that the extended guanidino could bind to the antagonist site in one conformation, but could also bind to the agonist site – still believed that the +ve charge was necessary At first, chemists thought that the strongly basic character of the guanidine group would be great. They envisioned this highly cationic species competing successfully with histamine’s cationic nitrogen (which has a pKa of 9.4 and would be less extensively cationic at any pH than the guanidine nitrogen). They erroneously thought that, once bound to the H2 receptor though this super-strong ion-ion bond, the rest of the structure would provide the antagonist activity they wanted. But they soon found out that these compounds were still H2 agonists because of that cationic group. Burimamide - - Needed to remove ALL agonist activity. The requirement for the antagonist to be charged was investigate - thiourea analogue (neutral not basic) was found to be a weak antagonist devoid of agonist activity. Thiourea group is very similar to guanidio group apart from pKa, both planar, similar in size and can take part in H-bonding. The thiourea group is neutral because the C=S has an electron withdrawing effect, pulling electrons away from the neighbouring nitrogens and therefore making them less attractive to H+, similar to amide nitrogens. This functional group is essentially neutral (and unionized) at pH 7.4. The activity of this compound suggests that the agonist binding site involves ionic bonds but the antagonist site involves co-valent bonds. Final chain extension - moved thiourea group closer to antagonist binding site Addition of a methyl to the terminal nitrogen increased hydrophobicity leading to burimamide – a highly specific competitive H2-histamine receptor antagonist. However activity was still too low for oral administration. Antagonist - - Focus turned to the imidazole group in an attempt to improve activity, pKa of imidazole ring in burimamide was found to be much higher than histamine More basic and therefore more likely to be ionised at physiological pH – if we want an antagonist to bind at the same site then it should be as close as possible in properties to agonist. Side chain of histamine has a –ve inductive effect – pulls electrons away from imidazole ring towards the protonated N Side chain of burimamide has no protonated N and therefore has a +ve inductive effect on imidazole ring - pushes electrons in to it – this means that it is more attractive to protons and more easily protonated. - - First isostere investigated was sulfur – it is electron withdrawing but about the same size as a CH2, similar bond angle – replace the carbon one away from imidazole ring – simply because a synthetic route was available – resulted in increased antagonist activity. Has more of an effect on N because it is closer, enhances this nitrogen’s desire for electrons, and it clings to that fickle imidazole double bond. The net result is promotion of the desired Nt-H tautomer, and high affinity for the H2 receptor. Antagonist - - - - - In an attempt to increase the desired Nt-H tautomer further a methyl group which as a +ve inductive effect was added close to the Nt - it pushes electrons in to the ring through bonds which causes the “tautomerizable” double bond to move in the direction of the electron flow. It is “caught in the current” of electron movement, and “lays down” towards N. Since N is forced to take the double bond, N must take the hydrogen. CH3 is most commonly found at this position and has been shown to provide selectivity for H2 receptors when added to the structure of the nonselective agonist histamine. If larger groups are used in H2 antagonists, the potency of the agent drops, possibly due to steric hindrance to receptor binding. The methyl did cause an increase in pKa to 6.6 – which is the same as imidazole itself indicating that the effects of the two side chains cancel each other out. The pHa of 6.8 means that the imidazole ring is 20 % protonated at pH 7.4, however the increase in the percentage of the tele tautomer outweighs the detrimental effct of the increase in pH. In effect the compound is “locked” in the form that is essential for receptor recognition but prohibited from forming the tautomeric form needed for receptor activation. They bind tightly to the H2 receptor, but can’t stimulate it. And this is just what we want in an H2 receptor antagonist. Electron-donating effect of methyl group is more significant at Nt Increases basicity of Nt Favours tautomer I over tautomer II Increase in pKa to 6.80 Increase in ionisation to 20% Increase in the population of tautomer (I) outweighs the increase in population of the ionised structures (III) Side chain effect on pka - - The pKa of the imidazole ring of histamine is 5.74, ie the ring is a weak base and mostly unionised at pH 7.4. The pKa of imidazole itself is 6.8 and burimamide is 7.25. Therefore burimamide is more likely to be ionised at physiological pH. This is because the side chain of histamine is electron withdrawing, resulting in a decrease in the pKa of the imidazole ring compared to the imidazole itself, where as the side chain of burimamide is slightly electron donating which results in an increase in the pKa of the imidazole ring. Therefore, the side chain of burimamide needed to be made more electron withdrawing, so that the proportion of unionised form is more similar to that of histamine – insertion of the electron withdrawing S lead to thiaburimamide. - - In histamine at physiological pH the major tautomer has the t-nitrogen protonated. To enhance the amount of tautomer I present in the antagonist, a methyl group was placed next to the N-t. The methyl group is electron donating and therefore makes the N-t more basic, but the Me group is also small enough not to cause steric interference with the receptor Histamine is approx 3% ionised at physiological pH, burimamide is 40% ionised at physiological pH Inductive side chain effects - - - Since the tele tautomer is important for histamine activity, it is reasonable to assume that the tele tautomer is also important for H2 antagonist activity, it has been suggested that H2antagonists undergo electronic rearrangement which induces a conformational change in the receptor The optimal position for the sulfur atom is attached to the first CH2 group of the moiety. If sulfur was attached directly to the imidazole ring, one of the lone pairs would orient itself in a perfectly parallel way with the electrons of the ring. In other words, there would be conjugation between the sulfur atom and the ring. If we push electrons into the imidazole ring from the R2 side, we’re blowing the electronic wind in the wrong direction. The “tautomerizable” double bond would be blown back toward N, and the NH tautomer would start to predominate. In other words, we’d start to lose the tautomeric form we need for receptor recognition and receptor binding. Antihistaminic activity would, therefore, also be lost. So the CH2 group that stands between the sulfur atom and the imdazole ring serves as the “brick wall” to resonance, but still keeps the sulfur atom close enough to the ring for its negative inductive effects to be felt by the double bond. It’s as good as it’s going to get in this system and, luckily for the patient, it’s good enough. Antagonist - - - Urea and guanidino both less active than metiamide less active than the guanidino But we know that the guanidino group is protonated as physiological pH – however with the 4 atom chain, the guanidino had only antagonist activity – possibly the longer chain length meant that the +ve charge was too far away from agonist binding site. Thought that toxicity was due to thiourea grp since this group is not naturally occuring, tried urea analogue but this was inactive, tried guanidine analogue, which was less active that metiamide. Although antag activity was weak, decided to look in to this further since guanidino group occurs in natural amino acids and therefore less likely to be toxic. Needed to find a way to make the basic guanidino group neutral to stop it being ionised at pH 7.4 Graph - In the case of guanidinos the pKa was found to be inversely proportional to the electonn withdrawing power of a substitutent. Very strong EWG such as nitrile and nitro pull the electrons away from the guanidine so that they are not available to attract a proton, decreasing the pKa Cimetidine - - - - - - The aromatic imidazole ring binds to H2 receptors through Van der Waals binding. It has basic character (pKa 6.8) due to the doubly bonded nitrogen, N. Cimetidine’s i:u ratio would be approximately 2x105:1 at pH 1.5, ensuring effective ion-ion anchoring to the anionic ASP98 of the H2 receptors in the parietal cell. At pH 7.4 the i:u ratio would be 1:4, ensuring good transport through biological membranes to reach those gastric H2 receptors.. R1 = CH3: The +Is group promotes the desired Nt-H tautomer that is essential for H2 receptor recognition R2 = ethylthiomethyl: This -Is group also promotes the Nt-H tautomeric form. It is approximately the same length as a butyl chain, which maintains the optimal separation of the aromatic ring and the cyanoguanidine group for high affinity binding to the H2 receptor. R3 = cyanoguanidine: This group is very polar, but neutral. The electron withdrawing cyano group destroys the basicity of the guanidine nitrogens, and they are unable to take on proton. The group is essentially 100% unionized at any pH, which promotes potent and pure H2 receptor antagonism. The NH groups of the guanidine are H-donors in H-bonds with ASP186 and ARG257 (or TYR182) residues on the H2 receptor. The terminal N-CH3 adds some lipophilic character, which enhances activity From a toxicity standpoint, cimetidine is the most problematic of all marketed H2 antagonists. This drug inhibits CYP450, leading to an increased potency and duration of action of co-administered drugs that are metabolized by the cytochrome enzyme system. The imidazole ring is responsible for this activity. The basic imidazole nitrogen competes with a HIS residue of the CYP protein that normally complexes with the cytochrome heme Fe+2, causing reversible inhibition of several isoforms, including CYP3A4. Several important drug-drug interactions have been documented. Use cimetidine with caution in patients taking other drugs metabolized by CYP enzymes. Cimetidine itself is metabolized by CYP450, with reactions including sulfoxidation and hydroxylation of the “benzylic”-like C4-CH3 group. All metabolites are inactive. Cimetidine also induces antiandrogenic side effects (gynecomastia, impotence), and is more likely than other H2 antagonists to cause CNS side effects (e.g. mental confusion) Conformations - Cimetidine has been shown to exist as a mixture of Z,E and E,Z isomers The double bond Z and E nomenclature is used here because this is a delocalised system and all the N-C bonds have double bond character. ZZ and EE forms are not favoured because of the steric interactions - Believed that the E,Z form is the active isomer – forms 2 H-bonds – one to the left and the second one downwards Dipole-dipole interactions - - Explored other functional groups for the polar hydrophobic end – found that the compounds with the greatest activity had an angle between the direction of the dipole moment and the NR bond (which is vertical in this diagram) of close to 30 degrees. Believed to be important for alignment of molecule as it approaches the receptors. In A – nitroketine aminal group of Famotidine has correct dipole and all functional groups are aligned with receptor in correct position to form H-bonds. In B – which is an imidaoline analogue used in the study, when the dipole is aligned the functional groups are in the wrong position for forming strong binding interactions. Other H2 antagonists - - - - - - Ranitidine The aromatic furan ring, coupled with the basic dimethylaminomethyl side chain, is considered isosteric with the aromatic and basic imidazole ring. The pKa of the sidechain amine is 8.2. Therefore the i;u ratio would be approximately 5x106:1 at gastric pH 1.5, and 6.3:1 at pH 7.4. Like the imidazole ring of cimetidine, the furan ring of ranitidine binds to the H2 receptor via Van der Waals forces. The protonated nitrogen of the dimethylaminomethyl sidechain binds this antagonist to the anionic ASP98 of the H2 receptor. The ethylthiomethyl connecting group is approximately equivalent in size to a butyl chain, and maintains the desired distance between the furan ring and the modified guanidine group. The electron withdrawing character of the ethylthiomethyl group is not important to us anymore since we don’t have an imidazole ring. The furan ring cannot tautomerize! R3 is a diaminonitroethene group, which is very polar, but non-ionic. Potent and pure H2 antagonism is observed. The therapeutic advantages of ranitidine over cimetidine include: A 10 fold higher potency A longer duration of action The lack of an imidazole ring which greatly limits CYP450 inhibition. However ranitidine can still inhibit some CYP isoforms including 3A4, 2D6 and 3A3. The inhibition is not as extensive as with cimetidine, and very few drug-drug interactions are clinically significant. Ranitidine is excreted largely unchanged, although some N-dealkylation and S-oxidation can occur. None of the metabolites are active. Famotidine The guanidine group on the aromatic thiazole ring provides the basic center and makes the moiety isosteric with imidazole. The thiazole ring binds to the H2 receptor via Van der Waals forces, and the thiazole C2-guanidine group, in cationic conjugate acid form, anchors to the receptor ASP98. The pKa of famotidine is 6.6. Therefore, the i:u ratio would be approximately 1.3x105:1 at gastric pH 1.5, and approximately 1:6.3 at pH 7.4 Note that the ethylthiomethyl group is still with us! As with famotidine, it is needed to keep the aromatic thiazole ring and the modified guanidine group separated by the proper - - - - - - - distance and, possibly, to properly orient these groups for optimal binding to the H2 receptor. The thiazole ring cannot tautomerize, therefore the electron withdrawing properties of the sulfur are not believed to be important to us here. The sidechain guanidine group (where cations fear to tread) has been modified with an electron withdrawing sulfonamide. Like the cyano and nitro groups, this group destroys the basicity of the guanidine, and prevents the formation of cations in this area of the structure. Potent and pure H2 antagonism results. The potency of famotidine is approximately 40-60 times that of cimetidine and 9-15 times that of ranitidine. Note that there is no terminal CH3 group on the modified terminal guanidine moiety (which would probably have increased activity even further). This H2 antagonist has a safety index along the lines of ranitidine. It does not inhibit CYP450 so few drug-drug interactions are observed. Like other H2 antagonists that increase gastric pH, famotidine may influence the bioavailability of co-administered drugs that require strict pH control for effective and controlled absorption (cephalosporins, iron, ketoconazole, etc.). We’ll see the same drug-drug interaction potential with the proton pump inhibitors. Nizatidine Nizatidine is a hybrid of famotidine and ranitidine. It has the aromatic thiazole ring of famotidine, and the diaminonitroethene and dimethylaminomethylgroups found on ranitidine. Even though it has the same basic dimethylaminomethyl group as ranitidine, its pKa is lower (6.8 vs. 8.2) since two thiazole heteroatoms (N and S) will pull electron density away from the lone pair of electrons of the sidechain amino nitrogen, rather that the one aromatic heteroatom (O) of the furan ring of ranitidine. The electron pull of these heteroatoms is through bonds. Not surprisingly, nizatidine has an H2 antagonist activity similar to ranitidine, but it is much more bioavailable after po administration. However, an identical dosing schedule of 300 mg/day is used. The antihistaminic potency of nizatidine is 5-18 times that of cimetidine. Like famotidine, nizatidine does not inhibit CYP450. Unlike ranitidine, the N-desmethyl metabolite retains some H2 antagonist action. Attendance question decreased white blood cell count H1 vs H2 antagonists - - - The imidazole ring, which is found on the agonist (histamine) and in one antagonist (cimetidine), is not essential in and of itself. However, the critical chemical features of this ring (aromaticity and a basic nitrogen atom) are. We used the imidazole ring in the pharmacophore drawn above because it was initially thought to be essential. However, we will soon see that other aromatic systems can provide good antihistaminic action at H2 receptors as long as they have a basic functional group either incorporated into the ring or attached to it as a substituent. Again, the basic group is essential because it is believed to protonate at the acidic pH of the parietal cell and anchor the antagonist to the anionic ASP98 residue on the H2 receptor. Any aromatic system used in an H2 antagonist must be isosteric with the basic imidazole ring in order to be effective in inhibiting histamine’s effects at H2 receptors. - If the imidazole ring is present, its structure must promote and maintain the Nt-H tautomer for receptor recognition. The drug must bind to the H2 receptor before it can antagonize it. However, we do not want to promote tautomerization because we do not want to stimulate the receptor. Remember, it’s the NH that has the right chemistry for receptor activation. We don’t want this property in our H2 receptor antagonists. - Two of the three “R” groups of the H2 antagonist pharmacophore serve to stabilize imidazole ring-containing H2 antagonist structures in this most important NH tautomeric form. They are: Histamine binding site - 191ASP 98 should say 198 Revision of histamine binding at the histamine receptor So what you end up with is an ion-dipole bond between the Asparagine 198 which now has a positive charge and a H-bond between the proton on the pi nitrogen and the amine of Lysine Proposed binding of an antagonist - ASP 98 should say 198 From our SAR discussions thus far, we can see that, compared to the agonist histamine, H2 antagonists do a “flip-flop” at the receptor. The basic functional group of the aromatic ring of the H2 antagonists (in cationic acid form) binds to the ASP198 that originally bound the sidechain of histamine. In a similar fashion, the sidechain of the antagonists binds to the receptor area [ASP186 and ARG257 (or TYR182)] that originally bound the nitrogen atoms of the aromatic imidazole ring of histamine. As an analogy, both agonist (histamine) and antagonist lie in the same bed (but not at the same time!) but one likes to sleep with its head on the pillow while the other places its head at the foot of the bed. Proposed binding of an antagonist (2) - Similar to histamine, there are Van der Waals interactions between the pi electrons of the imidazole ring or other aromatice ring in this case furan and aromatic amino acid H2 – antagonists bind at the same site as histamine, - just the other way around – they directly prevent histamine from binding – they are competitive antagonists. Compare and contrast - If you compare H1 antagonists with H2 antagonists, - remember that H1 antagonists are not competitive antagonists they bind at a different site on the receptor and cause a conformational change that prevents histamine from binding.