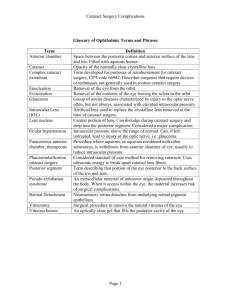
Crystalline lens Anatomy
advertisement

Crystalline lens Anatomy Shape o The lens is biconvex in shape. o The anterior surface has a different curvature than the posterior. It is flatter at 10mm, vs. the posterior surface, which is about 6mm when unaccommodated and focused at infinity. It is also less curved in the periphery, reducing spherical aberrations. Curvature also flattens anteriorly with age. o The circumference around the edge of the lens is the lenticular equator. It has a notched, crenated edge due to zonular attachment and pulling. o When not accommodated, the largest diameter of the lens is 9-10 mm. o At the geometric center of the anterior surface is the anterior pole. Similarly, the posterior pole is at the geometric center of the posterior surface. A theoretical line joining the two poles is called the lenticular axis. The distance between these two poles, or the thickness, is 4-5mm. o With accommodation, the lens becomes more spherical, decreasing the diameter and increasing the thickness. Placement o In the orbit the lens is just posterior to the iris and anterior to the vitreous body. The lens makes contact with the iris and aqueous body anteriorly and the vitreous posteriorly. o The lens is suspended by zonules (suspensary ligaments), attached to the ciliary body. o Spaces Circumlental Space- 0.5mm between the equator of the lens and the ciliary body. Capillary Space of Berger- a potential space behind the lens against the vitreous. Microscopic Anatomy o Capsule The capsule is a thin, transparent envelope surrounding the lens made of type IV collagen fibers. There is no elasticity, because there are no elastic fibers. o It becomes less elastic with age. Composition o 10% GAGS o 4% Heparin Sulfate Decreases with age For transparency? The capsule is pretty tough. Nowadays, it is left in place with cataract surgery. When capsule is extracted, it needs to be frozen and cut. o Histologically, it is a very thick basement membrane. It is secreted by anterior epithelium (anterior) and superficial fiber cells of lens substance (posterior). o It is constantly being regenerated. The capsule is unusually thick, at about 13 microns, but this varies between regions. The thinnest part is at the posterior pole. o 4x thinner than anterior pole On both the anterior and posterior surface is a circular band of maximal thickness. The circular zones are concentric with the lenticular poles. o The anterior (21 microns thick) is 3mm from the pole. o The posterior (23 microns) is 4mm from the pole. o These bands are thought to determine the spherical shape of the lens during accommodation. Two zones in cross section Outer- Zonular Lamella o A very thin membrane surrounding the capsule in which the zonules attach. Inner- Cuticular Aspect o This is the thicker cuticular proper. o It is composed of 30-40 lamella, each of which itself is made up of fine collagen fibers. Anterior epithelium The anterior epithelium is a single layer of cuboidal epithelium. It extends from the anterior poles to the equator. Typical cells of the lens have all the organelles that cells in the other parts of the body have. o The apex faces anterior, and the base is next to the capsule. Due to cuboidal dipping into spaces between the epithelial cells, the lens can have a dimpled appearance, called lenticular shagreen. The shape of the epithelial cells varies according to their location o The basal portion of the cell extends posteriorly along the inner surface of the lens capsule while the apical end of the cell slips behind the anterior epithelial monolayer. As the lengthening cells are pushed inward the flattened nuclei move anterioly. These nuclei form a crescent-shaped pattern at the equator called the lens bow. As the cells move to the interior, they elongate, eventually lose their nuclei and become lens fibers. Based on level of metabolic and mitotic activity, the anterior epithelium can be divided into three regions. Central Zone o These cells are near the anterior pole. They are the most cuboidal, being short and broad. o This region has the slowest rate of metabolism. Intermediate Zone o This area is between the anterior pole and equator. o The cells begin to elongate and a little faster metabolism. o Equatorial Zone o These cells are the most elongated as they are pushed into the interior of the cell. o It is the zone of highest metabolism of all cells in the lens. Lens substance is produced continuously. In the germinative zone, 100 cells/hr undergo mitosis. They then migrate to the lens bow. The lens grows throughout the entire life of the individual. This is perhaps the cause of presbyopia. Tight junctions These are located near the apex in the many infoldings and interdigitations between the cells. NaK pumps are also found between the cells. Lens substance The lens substance is composed entirely of lens fibers, the lens substance that makes up the bulk of the lens. It is produced by the equatorial zone cells of the anterior epithelium. The lens fibers are elongated, tapering cells which form a large U as they stretch from the anterior of the anterior pole to the posterior pole. Internal organelles lost, except for ribosomes. This is good for transparency. Cross Section The fibers are regular and hexagonal in shape, measuring about 7 microns wide and 5 microns thick. The lenses are densely packed. They are interdigitated to fit together and are also held together via a cement substance so that there are no spaces. Yet, movement is allowed. These connections are thought to retain the arrangement of the fibers during accommodation. These are extensive gap junctions that enhance communication so that the lens can function as a syncytium, which is important to the metabolism and physiology of an avascular tissue. The uniformity of the lens arrangement causes destructive interference. Lamellar Nature of Lens This is laid during embryonic development. Because the lens produces lens fibers throughout life and the older fibers are pushed to the center, the lens substance has a laminated organization. The optical discontinuity from cortex to nucleus allows identification of the nucleus with the biomicroscope. Nucleus o The older, more compressed fibers form the nucleus, at the center. This makes the optical density greater than that of the cortex. Fibers in the nucleus are more sclerosed. o This can be broken down into further layers based on developmental zones. The embryonic nucleus is seen at the center of the lens. This is formed first in development at 1-3 months. The fetal nucleus is formed at 3-8 months. This is what is identified by the Y sutures. After birth, the adult nucleus is formed. This is identified by the stellate sutures. This nucleus increases in size throughout life. Cortex o The newer fibers form the cortex, a layer surrounding the nucleus. Cortical fibers are considered to be softer. The youngest have not yet lost their nuclei and cytoplasmic organelles. Sutures due to lens growth Instead of meeting at one point, the fibers meet in close proximity to each other near the poles along suture lines. The pattern of polar suture lines becomes more complex as the lens develops. Simplest of the patterns is the Y-shaped sutures on the fetal nucleus in the center of the lens. The anterior Y is erect and the posterior Y suture is inverted. Later fibers develop further away from the center, and the subsequent sutures are of a more complicated stellate pattern. These sutures are used to identify the nucleus. Cataracts can occur in these sutures, called sutural cataracts. They are opacifications with no vision obstruction. Composition o This differs with age. o Water- 66% o Protein- 33% This is twice the amount of proteins than found in most body tissues. This allows for an increased index of refraction. Two types, depending on their solubility in water. Water soluble- the Crystallins (85%) o Alpha, beta or gamma- defined based on chromatography (size) o Alpha 35% of the lens protein Molecular weight of 800,000 to a million Daltons (large) 4 different subunits- A1, A2, B1, and B2 Each has a molecular weight of about 20,000D. They are held together by hydrogen bonds and hydrophobic interactions. A2 and B2 are direct products of gene translation. A1 and B1 are modified after gene translation by removing an amino group from A2 or B2. o o Beta A1 and B1 are only present in the fibers and not the epithelium, because they are not a direct product of translation. 55% of the lens proteins Two major fractions- beta (low) and beta (high), based on weight Beta (low)- MW of 40,000 to 90,000 Daltons Beta (high)- MW of 100,000 to 200,000 Daltons Gamma 1.5% of the lens proteins MW of 20,000 Daltons o HM = High Molecular Weight 5,000,000 Daltons Due to age, the crystallins clump to form large aggregates of alpha, beta, and gamma components. This crosslinking makes the proteins more insoluble and is a precursor to cataracts. Water insoluble- Albuminoid (15%) o Initially discovered in 1894 by Morner, these were not studied much until Dische (1965), who discovered that the water insoluble proteins could be separated into two portions: that which dissolved in 8M urea, and that which did not. o The urea insoluble proteins make up most of the membrane proteins. o One protein makes up 55% of all membrane protein- Main Intrinsic Protein (MIP). MIP has a molecular weight of 26,000 Daltons, which is cleaved to 22,000 Daltons after translation. It is only found in lens fibers, not in the epithelial cells. It is concentrated in the gap junctions, so it must be an important protein involved with communication. Structure of Proteins α-helix o This is a spiral form of proteins in which the proteins are made of individual amino acids. o With large R groups, there is light scatter, so this conformation is bad. None of the crystallins form alpha helices. Only betaconformations. β-conformation o Proteins are held together by hydrogen bonds and hydrophobicity. R groups are all located above, therefore there is decreased light scatter. o Functions Microfilaments (actin) and microtubules (tubulin) maintain the shape and aid in accommodation. Stress fibers (tropomyosin, myosin, d-actin) are seen with stress and repair, therefore are located in older cells. Beaded chain filaments o Backbone proteins o Since these are only in the lens, they can possibly cause autoimmune reactions if released in the body. Immune isolation Due to the suspension of the lens and lack of vascular supply, if the lens protein is released into the eye, an immune reaction can occur. This plugs up the trabecular meshwork, leading to an increased IOP and glaucoma. It can occur via cataract surgery if the lens is broken or in a hypermature cataract if not removed. The protein liquefies and the capsule can leak. Lipids, electrolytes, and carbohydrates- 1% Lipids Cholesterol- 50-60% o Increased cholesterol means increased liquidity of the lens fibers. Glycosphingolipids o In interdigitations o For communication? Phospholipids o Many, but the most common is sphingomyelin. With age, the concentration changes. o Some increase, and some decrease. With increased age, cholesterol and sphingomyelin double, therefore there is more rigidity and easily broken. o It is unsure if this is a precursor to cataracts. Zonules Function The zonules are the suspensory fibers passing from the ciliary body to the equatorial area of the lens. They function in holding the lens in place, as well as playing a role in accommodation. Anatomy Formed from the condensation of the tertiary vitreous o Not elastic or fibrous o Made of collagen-like glycoprotein, but they are noncollagenous. o Acidic mucopolysaccharide chondroiton sulfate o 5-30 microns in diameter, with each fiber banding every 12-14 nanometers. Attachment: Ciliary body to capsule o Originate from both the pars plana and pars plicata of the ciliary body. How the zonules attach to the ciliary body is not completely understood but electron microscopy shows most zonules appear to originate as small fibers blending into the basement membrane of the nonpigmented epithelium. Some suggest the zonules penetrate deeper into the area of the base of the pigmented epithelial cells in the valley between the ciliary processes. o Insertion of the zonules is into the lens capsule about 2.5 mm wide, which extends concentrically around the equator. The zonules form anterior and posterior sheets, the anterior inserting 1.5mm in front of the equator, and the posterior zonules terminating 1mm behind the equator. A few zonules insert at the equator. Actual insertion of the zonules is into the zonular lamella of the capsule. Before inserting the zonules appear to break up into finer fibrils and extend over the capsular surface, then join the zonular lamella. Ciliary Attachment o Most go to pars plicata o Some go to pars plana Lenticular Attachment o 1.5mm anterior to equator to 1.0mm posterior o Some are attached directly to the equator Potential Spaces o Posterior zonule surface and anterior vitreous: Canal of Petit o Within Zonular “V”: Canal of Hanover There is some mixing of vitreous and aqueous here Classes of Fibers o Principal Fibers- suspend the lens Run from ciliary body to the lens capsule For accommodation Two groups- based on ciliary body attachment Orbiculocapsular- posterior ciliary body attachment to lens o Anterior to lens attachment: orbiculoanteriorcapsular Arise in posterior pars plana to anterior equator These are the thickest of zonules o Posterior attachment: orbicularposteriorcapsular Arise at ora serrata to posterior equator o Ciliocapsular- attach at ciliary processes to lens o Cilioposteriorcapsular Arise in valleys of processes to posterior of equator Most numerous o Cilioequatorialcapsular Attach at lens equator Arise at anterior ciliary processes Association Fibers- for accessory Maintain tension, shape, movement, etc. Structural- do not run to the capsule Orbiculociliary- run from pars plana to ciliary processes Interciliary- between ciliary processes Circular- connect from zonule to zonule o Make it look like a spiderweb Pathophysiology o If diseased, leads to ectolentis Physiology Functions of the lens o Refractive The lens represents 33% of the eye’s power. The cornea is the other 67%. It has a power of +20 in the eye and +60 outside of the eye. Spherical aberration reduction mechanisms Asphericity Gradient index o The index of refraction is 1.39, but it is higher in the center, because that is where it is the most dense. o Transparency/ Maintain clarity The lens is transparent to light, because all internal structures are smaller than ½ the wavelength of light. This transparency changes with age. Someone at 80 years of age needs 3x more light than younger patients. . o Protection of the retina Most UV (light <400nm) is absorbed and cut off by the lens. UV can cause damage. There is about a 5% transmission. The lens absorbs UV more after 15 years of age, so it is very important for babies to have some form of sun protection. o Accommodation The young lens is a pliable mass surrounded by an elastic capsule. When the lens is focused on a distant object, the ciliary body is relaxed. In this state, the ciliary body has moved posteriorly, causing tension on the zonules, which increases the lenticular diameter and decreases the anterior-posterior dimension along the axis. When the ciliary muscle contracts (decreased tension on zonules), the lens becomes more spherical, thus increasing its dioptric power by 12-15 diopters. The front surface of the lens changes shape. The lens moves forward slightly, decreasing the depth of the anterior chamber. o This is seen with PS Images. o The center probably bulges out more than periphery, and the posterior is fixed. The lens sinks a little due to zonular release and gravity. The IOP rises momentarily and then decreases, caused by increased resistance at the lens/ iris interface, as well as the anterior chamber . The circumference of the equator of the lens decreases The thickness increases, and the diameter decreases. The stimulus to accommodation is blur. Pathway o Afferent Blur detected by the retina Retina LGN Striate Cortex Area 19 (stimulated = accommodation) o Efferent Parasympathetic Edinger-Westphal Nucleus exits the CN III Ciliary Ganglion 97% fibers go to ciliary muscle (3% go to sphincter) o The role is largely passive. The accommodative response requires about 350 msec to initiate o This is a long time due to the pathway. o An advantage of the long latency is that accommodation can be halted at any time. Depth of focus o A decreased pupil size during accommodation increases the depth of focus. o More DOF just enough to encompass the stimulus. o Dark Focus- 1.7D accommodation in complete darkness suggests equal innervation (sympathetic and parasympathetic). o Some with Pilocarpine (affects sympathetic). o But no anatomical demonstration of sympathetic innervation Accommodation ability is a function of age. Presbyopia begins around age 40-45 with a decrease in the accommodative amplitude of the lens. it decreases about 0.3D per year. All accommodation is lost by the age of 50. There is still a depth of focus of about 1-2D. Potential causes of presbyopia o Weakening of the ciliary muscle This really cannot be accounted for since as kids there is decreased muscle, and it increases with age. Yet kids can still accommodate. o Hardening of the lens Due to increased fibers, there is increased rigidity. o Enlargement of the lens Yet, zonule length is constant, so tension is released. This does not explain the change in prescription. o Loss of capsule elasticity This is also true, but how does it affect accommodation? o It seems that these all have an effect. Biochemistry o Kinsey and Reddy (1965) hypothesized the existence of the “pump-leak” system. This is the same as the cornea. The pump is the Na+/K+ ATPase, and it takes out waste. The “leak” brings in nutrition (oxygen and nutrients) from the aqueous humor. o The epithelium is responsible for the transport of materials into the lens The NaK ATPase pumps on the lateral surfaces of the epithelial membrane cells maintain the dehydrated state of the lens. Na is pumped out. Water follows. K is pumped in. All of this requires energy in the form of ATP. Na+ flows to the vitreous from the aqueous and enters the lens down the concentration gradient on the posterior surface. K+ is the opposite. o o Other Components Glucose Glucose enters the lens by facilitated diffusion. This is important with diabetes and cataracts. Lactic Acid This is the end product of anaerobic metabolism. Because the lens is anaerobic, this is pumped out. Glutathione Glutathione is synthesized by the lens. It consists of three amino acids and is involved with lens repair. Ascorbic acid and Vitamin E These antioxidants protect the cells of the lens by removing free radicals. This is pumped in. Inositol This is pumped in and is collected by the lens. The function is unknown. Amino acids These are also pumped in and are actively collected by the lens. They have the ability to diffuse out. In the lens, the amino acids make proteins and can be metabolized for energy. Energy Metabolism Glucose brought into the lens via facilitated diffusion Glucose consumption by the lens is 3-5mg/day. Oxygen consumption by the lens is 0.1ml/mg dry weight/hr. o This is not a lot. The lens can survive anaerobically with enough glucose. Glucose has a constant diffusion concentration of 10mg glucose per 100g lens. This concentration is maintained until the aqueous concentration reaches about 175mg/100ml, as seen in diabetes. At this point, the sorbitol pathway is activated. 80% of glucose is converted to lactic acid (anaerobic). Most of the glucose is converted to glucose-6-phosphate. o At this point, it cannot diffuse out of the lens. o It is the rate limiting step, being 70-1000 times slower than all other conversions in the lens. This step would alter osmolarity. 2 Energy Pathways Anaerobic Glycolysis o This produces 75% of the energy of the lens. o Glucose-6-phosphate is converted to lactate. o 2 ATPs go in, and 4 ATPs come out, producing a net 2 ATPs. Aerobic Glycolysis (TCA cycle) o From 1 glucose molecule, 34 ATPs are produced. +2 from glycolysis, for a total of 36. o This can produce up to 25% of the energy needs of the lens, from 3% of the glucose in the lens Hexose Monophosphate Shunt or Pentose Phosphate Pathway 5% of glucose goes through this pathway o It is important in producing NADPH for fatty acid biosynthesis and the ribose needed in nucleotide synthesis. It also provides the NADPH needed for glutathione reductase and aldose reductase activity in the lens. Sorbitol Pathway Only active when glucose concentration high Sorbitol metablized to fructose, but at a slow rate, thus, sorbitol accumulates in the lens Increased concentration of sorbitol and fructose in the diabetic lens o Causes “Snow flake” cataracts in uncontrolled DM o Now entire lens becomes opaque. o Increased osmolarity pulls water in o Areas of sorbitol and fructose came in, pulling in water. o Opacities stay even when water leaves These draw water into the lens Same type of cataract seen in galactosemia (inherited) o Cant reduce galactose o Develop cataracts as infants o Here, galactose alcohol galactilol (dulcitol) Acts like sorbitol o Accumulates and results in cataracts Aldose reductase inhibitors (the enzyme which produces sorbitol) have been used in rats to stop cataract production o But don’t work in humans, because we don’t have a lot of aldose reductase. Sorbitol quickly reduced to fructose. Defects in energy metabolism result in cataracts Oxidative Damage and Protective mechanisms Free radical- an atom or atom group carrying an unpaired electron and no charge. Generally short lived. A free radical can take an enzyme or membrane protein and alter its structure. If it was an enzyme, then once altered, it can either no longer do what it was made to do initially or its not as effective. If it’s a membrane protein, its altered 3D structure and it could not cause scattering of light. Ex. O2- supraoxide anion Produced during normal cellular metabolic activity and by UV light. o Electron transport chain Highly reactive and can damage lens fibers, proteins, and DNA Damage can accumulate with time and lead to cataracts o Peroxidation of plasma membrane lipids can result in lens opacification o If not repaired, it is there for good because cells do not die. They are just added. DNA can be directly damaged and since none of the lens fibers are lost this could be very damaging (alters mitosis, etc) Glutathione helps to alleviate some of the damage resulting from free radicals Removal of O2o 2O2- + 2H+ (Cu, Zn, or Mn + superoxide dismutase) H2O2 +O2 H2O2 is just as harmful. o 2H2O2 (Iron + Catalase) 2H2O + O2 This occurs 5 million times per minute. o These reactions help glutathione Glutathione This is a repair protein Glutamic Acid + Cysteine + Glycine (G-C-G)- Small Found in reduced (GSH) and oxidized form (GSSG- glut. Disulfide) o 2P-S PSSP + H2 This is done by free radicals 2 proteins combined with sulfur are now altered, are not as effective, and increase light scattering. o 2GSH + PS-SP 2P-S + GSSG So proteins are repaired, but we have to do something about the glutathione, because we want to use it again. o GSSG + NADPH + H2 2GSH + NADP Via glutathione reductase Up to 500mg/kg of lens (large amount) The lens uses about 11% of its ATP to produce glutathione o The suphydryl groups of proteins are usually in the reduced state. Free radicals (which can be caused by UV light) oxidize the sulhydryl groups causing them to aggregate and lose their function. The two sulphydryls bond together. Two reduced glutathiones can donate their hydrogens to split the proteins (now proteins in reduced stat). Glutathione disulfide (GSSG) is then reduced by the NADPH produced by the hexose monophosphate shunt. Thus, the lens has repaired itself. Congenital and Developmental Anomalies of the Lens Congenital Aphakia This is the failure of lens development, either mechanical or congenital. It is very rare but when it occurs, it usually is seen with multiple other congenital ocular anomalies, i.e., microphthalmos. Coloboma This is a flattening or indentation of the lens circumference. It is usually located inferior (like retinal, choroidal, ONH, iris coloboma- not lid), because this is the last place of fusion. A coloboma is generally unilateral, and in this area there are no zonules or attachments to the ciliary body. Management o Watch for significant astigmatic refraction and astigmatic accommodation. This may cause amblyopia. o Watch for other congenital ocular anomalies such as cataracts, other colobomas, etc. Phacodyalisis This is the movement of the lens due to no lenticular support. Microspherophakia This is a small, round lens. It appears like a “pearl” when looking at it through the slit lamp. The lens is entirely visible upon dilation. Microspherophakia is very rare and bilateral. It is due to an interference with fibrogenesis. Long, lax zonules are visible. Microspherophakia produces a high myopia, ranging from -5 to -20. Inverse angle closure glaucoma is also possible. Glaucoma comes on during miosis. The pupil must be dilated to break. Associations o Isolated o Marfan’s Syndrome o Weill-Marchesani syndrome Short, small feet hands and neck o Homocystinuria Complications o Subluxation of lens (up and out in Marfans, down and in for homocystinuria) o Inverse angle closure Do not use miotics. Perform gonioscopy. A laser iridotomy may be needed. o Progressive shallowing of the anterior chamber Gonioscopy is necessary. Asymptomatic attacks are common. o Associated systemic disorders of Marfans, homocystinuria, etc. Treatment o Monitor thepatient every 6 months. Lenticonus/ Lentiglobus “Keratoconus of the lens” Lenticonus o This is an outward, conical-shaped distortion of either the anterior or posterior lens surface, although it is usually the anterior surface. o Lenticonus is very rare and is often bilateral. A distorted reflex is seen when performing retinoscopy. Lentiglobus o This is an outward, rounded bulging of the anterior or posterior lens surface. Diagnosis o Diagnosis is made on biomicroscopy, especially when dilation. o Diagnosis becomes difficult later in life due to increased opacification of the lens. o There is also significant internal astigmatism. Complications o If early onset, spectacle correction is necessary to prevent amblyopia. Management o If spectacle correction is inadequate or not possible, cataract surgery should be considered Ectopia Lentis Definitions o Subluxation is a lens that has moved, i.e., into the anterior chamber. o Ectopia lentis is a displaced lens that has fallen, i.e., it is gone, due to trauma, etc. Types o Hereditary ectopia lentis (without) associated systemic disease Simple ectopia lentis This can either be a congenital disorder or spontaneous disorder of late onset. When congenital, it is usually inherited as autosomal dominant without associated systemic abnormalities. It is usually bilateral, with symmetrical upward and temporal displacement of the lens. Ectopia lentis et papillae This is a rare congenital disorder in which lens anomalies are combined with pupillary displacement. It follows a recessive inheritance. The pupils are oval or slitshaped, ectopic, and dilate poorly. Due to the poor dilation, a B scan should be utilized to view the posterior pole. Bilateral microspherophakia is usually present. Cataract, glaucoma, and retinal detachment can occur. This is also seen with Peter’s Anomaly. o Systemic disorders commonly associated with ectopia lentis Marfan’s Syndrome This is an autosomal dominant condition in which ectopia lentis is present with skeletal, cardiovascular, and ocular anomalies. The primary defect involves the collagen. Skeletal defects include increased height, increased length of distal limbs (arachnodactyly), loose-jointedness, scoliosis, and anterior chest deformities. o o Cardiovascular defects include aortic silation, aortic aneurysms, and mitral valve disease that leads to a decreased life expectancy. Ocular anomalies o Amblyopia, high refractive errors (myopia), increased corneal diameter (megalocornea), smooth and velvety iris, miotic pupils that dilate poorly, angle abnormalities, and ectopia in 50-80% of patients. o Ectopia is usually bilateral, symmetrical (up and out), and nonprogressive. Homocysturnia This is an inborn metabolic error of sulfur-containing amino acids. It is an autosomal recessive condition with skeletal, cardiovascular, and ocular anomalies. Mental retardation is seen in 50% of patients. Ocular anomalies o Ectopia in 90% of patients o Subluxation is bilateral and symmetrical. The lens migrates inferior or inferior nasal. o Progressive dislocation can lead to pupillary block glaucoma. o Myopia is common. o Retinal detachment is a complication of lens surgery. o Treatment Monitor retinal changes. Perform visual fields and check IOP for glaucoma. Ocular disorders with ectopia lentis Trauma is the most common cause of ectopia lentis. Congenital glaucoma Rare Retinitis pigmentosa, aniridia, megalocornea, and Rieger’s syndrome Management of ectropia Children Emphasis is directed toward improving visual acuity at the earliest age possible and avoid complications of blurred vision in early life that can lead to refractive amblyopia. Advise against contact sports due to the high incidence of total dislocation and retinal detachment with trauma to head or eye. All patients should be referred for appropriate pediatric and medical consultation to search for any associated systemic disease. Cataract extraction Lens surgery is difficult and can lead to intraoperative and postoperative complications, because the zonules are not good. Indications for surgery include o Lens dislocation into the anterior chamber which can cause a reaction o Mature lens opacity o Lens-induced uveitis o Inadequate visual acuity o Imminent complete luxation of the lens Congenital Lens Opacities Remnants of tunica vasculosa lentis Mittendorf’s Dot o This is present in 10% of all children. Usually located inferior and nasal to the lens axis (off axis), it represents the point of attachment of the hyaloid system to the posterior capsule. A remnant of the hyaloid artery is common and can be seen as a fine white thread. o Detection Direct illumination with parallelepiped or optic section on slit lamp- white color. Retroilluminantion by direct ophthalmoscope or retro with slit lamp- small black dot o Significance No effect on vision o A white crescent at the former attachment of hyaloids artery is called Vogt’s reflex arc. Persistent Pupillary Membrane o These are found in 95% of newborns and 20% of adults. They are anterior epicapsular remnants of the tunica vasculosa lentis. o Appearance Fine grey-white filaments, found anywhere from the anterior capsule to the collarette of the iris. o Significance Rarely causes any blurring of vision, even if very dense Epicapsular Pigment Star o These are stellate pigment “stars” found on the anterior capsule, usually in the pupillary region. o Significance Even if dense, there is no effect on acuity. One must differentiate between pigment left on the capsule from trauma or uveitis vs. pigment stars. Retroiridal Pigment Lines o These are radially oriented fine pigment lines found on the anterior capsule. Dilation is needed to view, because they are usually peripheral to the pupillary region of the capsule. In the elderly, there may be zonular fibers inserting in the lens midperiphery with entrapped uveal pigment. o These are not significant as well. Congenital Lens Opacities/ Cataracts Congenital means that it is existing at or before birth. General characteristics o Congenital cataracts are often bilateral and are usually symmetric. o There is often no effect on vision, and they are non-progressive; however, 14% of all childhood blindness is due to this. o The location of the cataract indicates the time of development. Causes o Isolated hereditary (AD)- 20-25% o Rubella- 20% o Associated with other ocular anomalies- 5% o Sporadic, idiopathic- 33-50% Types o Capsular cataracts These are rare, small opacities in either the epithelium and/or capsule. Rarely are they axial, so there is no effect on vision. Tx: none o Polar cataracts These are opacities at the level of the subcapsular cortex. They can be at either the anterior or posterior pole, but the posterior pole is more common. They come in a variety of shapes, but are stationary. Vision is variable, but it is very often remarkably good. The posterior pole is more visually significant, though often there is no effect on vision. Flat discs in cortex are known as reduplication cataracts. Management/ Treatment If there is a significant effect on vision, try to dilate the pupils to see if vision improves. If there is no improvement with dilation or best refractive correction, cataract extraction should be considered. Watch for evidence of intra-uterine infection and uveitis. Strands to the pupil margin (white persistent pupillary membrane) will be seen. o Anterior axial embryonic cataract These are small white dots seen in the axial region near the Y suture in 20-30% of all children. There is no reduction in vision and no progression. o Anterior and Posterior Stellate (Sutural Cataracts) These are usually dense blue or white opacities located in the Y sutures. They are often bilateral and are usually familial (AD). They usually do not impair vision. o Zonular or Lamellar Cataracts These cataracts are due to brief exposure to cataractogenic substances or events during pregnancy, thus the lamellar (one to several lamellae of fibers) nature roughly dates the time of development. Usually the outer fetal nucleus or inner juvenile (adolescent) nucleus are affected. It appears like there are “oil droplets” in the lens. o Punctate This type of cataracts is the most common (25% of infants) type of cataracts. Small opaque dots of varying sizes and shapes are seen throughout the lens. These are stationary. They can be bluish and are termed cerulean cataracts or “blue dot” cataracts. It is extremely rare that these will affect vision. o Total Congenital Cataracts These are dense, white nuclear opacities. Rubella is the most common cause. Maternal transmission of the virus to fetus occurs during the 1st trimester. There is a 50% chance of congenital anomalies at this time. If the transmission occurs late in the 1st trimester, a very high percent will have cataracts. There is a classic syndrome of eye, ear, and heart defects. o Ocular anomalies include microphthalmus, shallow anterior chamber, miotic pupils, and salt and pepper fundus lesions (like syphilis). o Ear defects include hearing loss or deafness. o Heart defects include pulmonary stenosis, patent ductus arteriosus, and ventricular septum defects. Treatment/ Management o Refer to pediatrician. o Treat associated ocular conditions. Treat amblyopia if present (VT) Cataract extraction is needed Mydriatic agent if needed General Management o Few congenital cataracts produce vision loss, and they are rarely progressive. o If there is significant vision loss in early life, surgery is indicated to prevent deep amblyopia. The visual potential will otherwise not develop properly, leading to permanent vision loss. o Always carefully examine for associated ocular and/or systemic anomalies. Cataracts Introduction The word “cataract” comes from the Greek, “cataracta,” meaning waterfall. It was believed that fluids filled the lens, making it cloudy. A lens opacity that decreases either the quantity (VA) or quality (how you see) of vision. As of Nov 2008, cataracts will affect approximately 20.5 million Americans past the age of 40, with an expected increase to 29 million Americans by 2020. These are localized losses of transparency o Increases with age. Can be congenital o It is the number 1 cause of blindness In the world (third world) o There is a 50% chance that anyone over 75yo has a VA <20/30. o Anyone that lives long enough will develop a cataract Risk Factors o Age Advanced age is the primary risk for cataracts o Exposure to oxygen free radicals (ocidants Chemical toxins, cigarette smoke ot infections o Radiation and electromagnetic waves from sunlight and ultraviolet exposure Promoting oxidation and degradation of lens protein Association between UVB radiation and cataract formation o Medications Corticosteroids o Smoking Introduces oxidants to ocular tissues. Secondahand smoke exposure decreases zinc concentration in the lens. zinc is a natural antioxidant that plays a vital role in maintaining lenticular transparency and preventing lipid peroxidation. The accumulation of copper, lead and cadmium in the lens due to nicotine breakdown is associated with the hastening of cataractogenesis. o Alcohol Heavy alcohol consumption (4 or more drink a day) was associated with a higher incidence of nuclear cataracts. Classes o Naming is due to location of opacity (nuclear vs. cortical) o Congenital- found at birth (during embryological development) Already developed. Can be caused by Rubella. o Traumatic- a blow to the eye causes opaqueness It disturbs the cell structure of the lens o Acquired- result of normal aging Secondary to the oxidation process or free radicals Starts to affect when 60-70yo Everyone will get one UV might play a role DM can speed up the cataract formation Overdose on steroids also causes cataracts Aphakia is the removal of the crystalline lens One of the most important roles an optometrist can play in cataract comanagement is that of patient advocate. Helping a patient understand his options and then deciding what lens is best for him is critical to the patient’s success. Acquired Cataracts Cataractogenesis in Aging o Basis of lens transparency Lens scatters less than 5% of incident light due to Cell packing results in destructive interference of scattered light (regular array) Uniform refractive index throughout lens (increased slightly toward nucleus, but gradual) Gap junctions (electrical continuity) o All the cells that are produced by the lens remain throughout life. o Aging results in changes in these cells. Membrane phospholipids The different percentages of phospholipids in the cell membrane change as the cells age, resulting in a less fluid membrane o Sphingomyelin concentration doubles, therefore more rigid and easily broken. The MIP of the gap junctions is also cleaved from its 26,000 dalton posttranslational form to the 22,000 dalton form. Structural proteins are gradually lost The internal cell structure or the cytoskeleton of microtubules, actin and intermediate filaments is gradually degraded as the cell ages. Without a cytoskeleton, the cell has difficulty maintaining its shape. Old cells have less internal water and then shrink Crystalline proteins increase aggregation As the cell ages, the crystalline proteins begin to clump together o Increases index of refraction o Creates vacuoles (clumps of water) Initially the crystallins are strongly associated with water, binding about 24% of the water in the cell With aging, the tertiary structure of the proteins changes, due to pre and post-translational damage, and less water (13%) is bound by the proteins The reulst is clumps of protein and pools of water, referred to as syneresis Water content decreases When the water is no longer bound by the proteins, the osmolarity of the lens decreases and water flows out of the lens Cortical thickening 0.02mm/year Due to the increased thickness of the lens, the nuclear fibers may not be able to get the nourishment that they need from diffusion. o Increased cortex means increase distance to diffuse in and out. o Senile Cataract Changes Causes Xray, UV. IR, B rays, gamma ras, microwaves, free radicals Ultrasound, inhibitor of cholesterol synthesis, genetics, aging, DM, sugars Synthesis of glutathione decreases Since the concentration of glutathione decreases the structural damahge that results from UV and metabolic insult can not always be repaired (cascade effect) Ion pump becomes less effective Results in changes in the ionic concentration of the lens Increased sodium and decreased potassium and amino acids Results in less protein synthesis General decerase in metabolic activity Fewer proteins made, l;ess ATP available for normal functions (pumps, glutathione, and enzyme production) Crystalline proteins are oxidized and glycosylated (glucose added), leading to cross-linking and insolubility (increasing light scatter) Metabolic breakdown leads to osmotic imbalance, formation of vacuoles (increased concentration of water) and clefts (increased concebntration of protein). This changes the index of refraction and increased light scatter. Increased optical density leads to color changes (yellow, brunescence), especially in the nucleus. The decrease in the water content of the lens results in an increase in the optical density The lens nucleus changes colors because the breakdown of proteins. Membranes rupture and accumulation of debris results in opacities Osmotic pressure results in rupture of cell membranes This debris accumulates and scatters light and results in the cataract = bud formation. o Bud begins o Pinch off to inclusion bodies, leaving a hole in the membrane which is weak. o Bodies enlarge, increasing the osmotic stress o All membranes lost, decreasing destructive interference o Inclusion body enlarge and seen as a vacuole. Indications for referral for surgical removal of cataracts o Patient’s status and Visual Function Diminished visual function Prevetion of normal visual function and decrease in quality of life Loss of independence Inability to perform ADLs (activities of daily living) High visual demand Does this visual impairment both you enough to consider surgery? If the answer is no, then you should schedule a follow-up visit with the patient in about 6 months. If the answer is yes, then you should proceed with a referral for cataract removal and IOL implantation. o The standard requirement for insurance-reimbursed cataract removal is BCVA of 20/30 or woese, with required documentation of symptoms. Ocular health Inability to view retinal, ONH or macular structures when needed for management (i.e., DM, glaucoma, ARMD, etc) Prevention of detection and/or treatment of sight threatening disorders Medical indications Phacolytic glaucoma o Leakage of lens material (blockage of TM by macrophgases), leading to inflammation and increased macrophages. Phacomorphic glaucoma Phacoanaphylaxis glaucoma o Rare granulomatous reaction Lens-particle glaucoma o Lens material liberated by trauma or surgery that blocks outflow o + inflammation Dislocated lens Amblyopic secondary to congenital cataract Goals Patients should be motivated to have surgery (elective)- 90% Patient should have good education as to overall ocular health other than the cataract Poatient should be given all variable options Patient should be given time to generate questions and discuss with family members, friends, the decision to operate Patient should be given risk/benefit ratio of having the procedure performed. o Risk: infection (ophthalmitis)- 1 in 10,000 o Benefit: better vision Cataract Progression Sun (UV) Smoking Contraindications to Referral Procedure would not improve visual function or improve usage of low vision device by having surgery Amaurotic eye (blind) Longstanding detached macula/ retina Systemic health Patient should have a full physical prior to surgery o All cataract surgery patients should receive a routine physical examination. Consider based on patient’s systemic health = EKG, CBC, and chest xray. Cardiovascular, neurologic, problems which need to be handled first (unstable DM) Debilitating illness Any systemic disease which may increase risk factor of having surgery (CA, etc) o Ocular health Absolute o Neovascular glaucoma o Chronic active uveitis o Blind eye Only considered if the cataract is ausing the patient extreme pain or discomfort. Relative o Monocular patient o DR o POAG (IOP increases with surgery) o Decreased endothelial cell counts o Previous RD No lid disease (causes infection) Corneal endothelial disease o Because lose 500 cells with the procedure o We need 2500-3000 to function o Successfully corrected patient with spectacles/ contact lenses Mental Status Improving visual function would not improve patient’s life functioning (i.e., end stage Alzheimer’s patient) Mentally handicapped patient (same situation as above) Pre-Operative Ocular Evaluation Examination procedures Patient complaint/ History o Visual dysfunction = cataract problem (#1 goal) o Visual needs Administer a visual function survey or questionnaire. o Ocular history o Medical history Medications to ask about Anticoagulants o Aspirin, NSAIDs, gingko biloba, high doses of vitamin E. o These can increase the risk of bleeding during cataract surgery. o Stop taking all anticoagulant meds 1 week before surgery. They can resume them the next morning after surgery. o However, if someone has had a serious cardiac event or multiple strokes and is directed to be on aspirin by a doctor, the aspirin may not be stopped. o Note in records. o Stop Coumadin 2-3 days before surgery, unless there is a contraindication. Resume the day after surgery. Systemic alpha-adrenergic receptor antagonists or blockers. o Used to treat urinary symptoms of benign prostatic hypertrophy (BPH). o The most common drug in this class is tamulosin HCl (Flomax). o Others include: terazosin (Hytrin), doxazosin mesylate (Cardura), and alfuzosin (Uroxatral). o Can cause intraoperative floppy iris syndrome which results in an iris that billows in response to normal anterior chamber irrigation, progressive papillary constriction during surgery and a marked propensity for the iris to prolapsed to phaco and sideport incisions. . o Can also be caused by saw palmetto. o These do not need to be stopped, because studies show that damage that occurs to the iris muscle is permanent. So youre not going to gain any benefit from stopping the medication. o Current treatment includes identifying patients who have taken the drug, even many years before surgery, so that atropine canbe given preoperatively, along with intraoperative epinephrine. Chronic Narcotic Use o Causes permanently small pupil. o May need to intervene with hooks during surgery. Or use stronger dilating drops prior to surgery.can also use pupil expansion rings or high density viscoelastic agents. But these agents are difficult to remove and can cause higher pressure post-operatively. Diabetes increases the likelihood of leaking capillaries, and thus can lead to CME. o Family history o It is important to note eye turns, amblyopia, anisometropia, etc. Visual Acuity o Visual acuity is the single most important component of overall visual function. o Distance and near o Snellen Acuity, with pinhole o Under low and high illumination o Contrast sensitivity functions Potential Acuity o Laser/ White Light Interferometry Patient must be dilated Room illumination is moderate Explain to the patient about pattern of lines they will see. Set interferometer to “hi/ H” and target field to 8 degrees (at bottom). Set to 20.80 or larger if the patient’s BVA is worse than 20/80. Set it at one line above threshold. Can set orientation in any direction by turning the head of the instruments. Make sure you know the orientation before administering the test Test the better eye first so the patient understands the test Hold the instrument so the light is centered in the pupil and the cornea and iris are in sharo focus (you should see 3 slit light sources on the cornea oriented in the same direction of the pattern the patient sees) Ask the patient to indicate the orientation of the lines Continue to challenge the patient until they cannot get 2 out of 4 presentations This gives a more optimistic prediction than the PAM (overestimates) o Potential Acuity Meter (PAM) Patient must be dilated Attach PAM to the slit lamp and turn on Notch in PAM that fits onto metal rod by light housing hinge Position patient with refractive spherical equivalent dialed in Direct patient to close their eyes as you focus the light beam on the eyelid Patient should see the chart as they open their eyes and look down into the illuminated opening You may have to make slight horizontal, vertical, and inward movementsto get the optimal viewing for the patient. Give them time to adapt. Ask the patient if they can see numbers (they are two lines larger than letters) Have the patient work down the chart Push the patient until the test is completed to the best of their ability Underestimates the moderate-dense opacities and overestimates with certain macular conditions (CME, ARMD, serous detachments) Does not bypass opacities like interferometer. Glare Testing o Brightness Acuity Tester (BAT) Induces glare Patient is not dilated and wearing their best correction Room illumination should be dark Have the patient hold the BAT over the eye being tested (the other eye should be occluded) Turn the BAT to “med” and measure their distance snellen acuity Repeat with the BAT set to “high” 3 potential outcomes Acuity stays the same= no significant glare problems Acuity is reduced = glare is a problem for the patient Acuity improves = “pinhole effect” = recheck SRx. o Miller-Nadler (Titmus Optical)- utilized Landolt Cs (20/400) against constant sontrast background circle. The Cs vary in contrast to the background. Since the system employs a background glare with a contrast test, it may be useful in simulating daytime glare disability. o No uniform standards have been established for glare testing devices, but measuring glare is useful it correlates well with cataract symptomatology. Contrast Sensitivity o CSF testing evaluates the patient’s ability to perceive a variety of coarse, intermediate, or fine details at differing contrasts relative to the background. CSF testing is a more complete form of vision analysis than is Snellen testing. Two things are considered when measuring CSF The contrast threshold between the object/ background The target size of the object subtended on the retina and measure in cycles per degree o Some letter optotype charts are the Pelli-Robson, Regan and Terry. The patient reads letters of differing contrasts. The Regan uses varying letter sizes at varied contrasts, while the PelliRobson and Terry offer only one size letter. For these charts the room and chart illumination needs to be standardized. o Other automated testing devices B-VAT II (Mentor) Sine wave on computer CSV 1000 (Vector Vision) Sine wave on illuminated chart Eye Con5 (Eye Con) Letter on computer screen MCT 8000 (Vistech) Sine wave on table top viewbox Optech 100/2000 Sine wave on table top viewbox TVA (Innomed) Square wave on computer VCTS (Vistech) Sine wave on charts Pupils o Excellent neurological test o In order for the patient to achieve maximum success with apodized lens system, the pupil must be able to dilate in dim light and constrict in bright light. In general, pupillay function declines with age. o Colvard pupillometer may be obtained to determine mesopic pupil size. Cover Test/ EOMs o To rule out strabismus Color vision Keratometry o Data necessary for A-scan ultrasonography o Can estimate post-surgical cylinder o Very important for multifocal implants Refraction o Vital to avoiding unnecessary procedures Need a JND of about 0.75D o May need to PH best corrected refraction o Need BVA Biomicroscopy o Lid disease needs to be rules out Treat this aggressively. Even trace amounts of lid disease, namely blephartis, can have a profound and deleterious effect on subjective outcomes. Since lid disease can stifle appropriate tear production, lid disease should be managed aggressively prior to surgery. Lid scrubs bid Low dose tetracycline (20mg) bid Topical macrolide antibiotic suspended in a thick mucoadhesive Warm compresses bid with massage to express the thick secretions blocking the glands Cyclosporine Dry eye should be treated as well o Corneal health and endothelial function o Pachymetry o Corneal Topography Lenticular or corneal astigmatism Also reveals subclinical corneal anomalies Can also assess lid position pre and post-op. o AC estimation and clarity o Iris transillumination and gonioscopy Determine if there is any form of pigment sdispersion syndrome. o o Type of cataract- visual dysfunction Grade the cataract Oxford classification LOCS II (Lens Opacities Calssification System II) 1-4+ Scale Diagram findings o Can photograph o Anterior vitreous evaluation. Clear? Endothelial Cell Count o Need an electron microscope o Specular endothelial photography o Patients with signs of corneal edema, guttate or secondary implant candidates o Essential to prevent or predict post-op bullous keratopathy o Endothelial cell photograph is most accurate o Cell counts should be taken of the superior, central and inferior corneal endothelium o Cell counts below 800 cells/mm2 are at the highest risk to develop post-op bullous keratopathy. Anyone with greater than 2600 cells/mm2 will be fine. o The normal endothelium has an average cell count of 2200 to 2400 vs. abnormal endothelium, which has a low cell count of 1200 to 1500. Tonometry o Rule out pre-existing glaucoma Visual fields o Screening indicated o Cataracts cause a generalized depression but may cause any type of field defect Do 40 point screeners Dilated Fundus Exam o Rule out peripheral retinal anomalies which may cause postoperative RD risk o Rule out macular disease Rule out presence of occult epiretinal membrane and macular thickening. Subtle macular pathology, even fine pigment mottling, can negatively affect elective IOL outcomes. o Allow potential acuity assessment o Check vitreous fro anomalous adhesions (opacities or PVD) These may influence a patient’s subjective evaluation of outcome. Many patients erroneously believe cataract surgery will eliminate floaters. These will not disappear after cataract surgery. o Whatever the patient sees, the doctor see If the patient sees 20/40, the doctor sees 20/40 while looking at the retina. o If you find any retinal disease, treat it first, and then consider referring the patient for cataract procedure. A-Scan Ultrasonography (Echography) o Measures the axial length An error of 1mm in the measurement can create an error of 3D in the final IOL calculation. o This sends an echo in and records out. o Must identify true difference in axial length o Avoid corneal compression o Correctly identify retinal/ scleral spike vs. orbital fat o Look for the longest/ best scan for axial length. Measurement critical so it does not create myopia/ hyperopia. o Spikes are due to changes in n. o The A-scan provides a one-dimensional display of returning echoes in the form of vertical spikes of various heights and distances from the initial signal. o A-scan is the most commonly used technique to measure the axial length of the eye so the IOL can be properly calculated. o Each spike on the graph represents an ultrasonic echo from a specific ocular tissue area. Five principal echoes from the following structures are typically present: cornea, anterior lens, posterior lens, retina, and scleral/ orbital fat o Taking your measurement (contact method) 2 methods are possible Applanation o The probe is mounted on an applanation device (applanation tonometer biprism holder), on the slit lamp. After the cornea is anesthetized, the probe is brought into contact with the cornea by the use of the joystick, with application of minimal pressure. Free-standing o Position the patient sitting upright in the exam chair. After the cornea is anesthetized, place the top of the probe perpendicular to and in light contact with the corneal apex. Attempt to align the probe tip with the optical axis, avoiding pressure that could create corneal compression. You will get a continuous emission of ultrasonic waves, signified by a repeated “beeping” sound. With proper corneal alignment, the screen should display approximately equal spike heights for the cornea, anterior and posterior crystalline lens, and retina. The rise of the retinal spike must be reasonably sharp at 85-90 degrees off the baseline. Once there is spike equality and reading acceptability, there is a long beep and image is “frozen.” Take at least three readings that are within 0.15mm of each other and average them to help ensure accuracy. Make sure that the anterior chamber depth (ACD) measurements are consistent between readings to ensure against corneal compression. o Contraindications/ Compliocations This is a non-invasice technique so minimal risk is involved. Repetition with consistent readings is the key to success. Caution should be exercised to prvent corneal compression with the probe. The hand-held method increases the risk of corneal compression and improper alignment. Small pupils accompanied by significant lens opacification may require pupil dilation if initial testing results are poor. 0.3mm error in axial length results in a 1.0D error in IOL power. Consider taking more measurements when The axial length is less than 22mm or more than 25mm There is 0.3mm difference in axial length between eyes With uncooperative or poorly fixating patients IOL Calculation (Biometry) o SRK Formula P = A – 25L – 0.9K A = Garbage Constant o This takes into account how we use the keratometer, calibration, technique, etc. L = Axial Length K = Avg K Readings o Generally patients are given a -1 prescription for happiness and seeing. o PC/ Iris Fixated/ AC IOL Power > Aphakic SRx PC power has to be the stronger, because it is closest to the retina. There is a decreased focal length. Ophthalmic lenses are the weakest. With mistaken hyperopia, everything is blurred. This is called “hyperopic surprise.” A scan orbital fat spike is misidentified as the retinal or scleral spike, resulting in a lower IOL power. This is sometimes chosen for various reasons. o This needs to be done especially more carefully with patients that have had a previous refractive surgeries. Look for presurgical Ks. o It is imperative that you perform accurate pre=op biometry and use a nomogram, such as the clinical history method, that has been most successful for the multifocal in which the ametropic patient is interested. This minimizes the chance of a “refractive miss.” o When a patient undergoes refractive surgery, the shape and length of the cornea changes, making pre-cornea’s curvature obsolete. As a result, when these patients present for either accommodative or multifocal IOL implantation, you must carefully calculate the patient’s corneal refractive power. Do this by using a nonogram to ensure that patient’s IOL has the appropriate power. For patients who are candidates for a toric IOL, a computer generated Toric Calculator is used to determine the precise planned axis of rotation of the implant for optimal acuity (www.acrysoftoriccalculator.com). Topography or Pentacam Corneal Analysis Special consideration must be given to corneal astigmatism. The presence or absence of corneal cylinder can influence proper IOL recommendation. Asymmetric, non-orthogonal, irregular or apical astigmatism may confound candidacy for advanced IOL technology altogether. Aberrometry Aberropia is vision loss exclusively due to higher-order aberrations and not refractive power. Aberropia can prevent patient from obtaining optimal vision from their lenses. Emply an aberropmeter on these patients prior to cataract surgery. Mature Cataract Evaluation Where cataract is so dense that the patient cannot see out and you cannot see in with your brightest BIO illumination Things to consider when doing the patient evaluation o Ocular history o NLP? (determine retinal function) o Color perception (macula and papillomacular bundle are very sensitive to red) o Entopic Images (Purkinje Vascular Tree) Use a transilluminator and place it against the upper lid (eyes are closed) Ask the patient what they can see (no clues!) Patient should say “veins on a leaf” or “cracked mud” appearance 80% of normals note appearance. Positive result is a good indication of macular function, while a negative result is a poor indication of macular function o Maddox Rod o o Occlude the eye not tested Have the patient hold the Maddox rod over the eye to be tested in a horizontal or vertical position Hold a penlight/ transilluminator along the visual axis Ask the patient what they see and is the line whole or broken? A visible whole lineindicated a functioning macula and if the line is broken or wavy, there may be leakage in the macula or a macular hole Watsky Allen Test With a DFE and 78 or 90D lens, ask the patient what do you see right away? A white line means there is macular function Wavy means there is macular edema Broken indicates a hole B Scan Ultrasonography B-scan ophthalmic ultrasonography (echography) is a diagnostic procedure used for the detection and differentiation of ocular and orbital disorders. The B-scan (B for brightness) produces two-dimensional images, composed by coalescing dots of varying degrees of brightness. The horizontal axis of the echogram represents tissue depth, and the vertical axis represents the scanned segment of the globe or orbit. The focused beam’s reflection or echo is represented as a dot on the screen with it’s strength indicated by it’s brightness. The stronger the echo, the brighter the projected dot. Technique Clean the probe tip with alcohol and dry Spread the transmission gel on the transducer membrane of the probe Instruct the patient to close their eye Gently touch the probe to the closed lit and adjust the grain control (to enhance the image) Scan the eye by varying the position and angle of the pribe When a desired image appears on the screen, it can be frozen and printed. Contraindications. Complications B-scan is avoided on an eye that has had recent intraocular surgery, or may have a scleral laceration or a perforating injury Pre-op Meds Discussions before surgery Discuss your findings with both the patient and the surgeon. Informed consent document Inform your patient of the potential complications associated with the surgery Outline all post-op lifestyle limitations, such as avoiding heavy lifting, swimming, or contact with chemicals, and review a list of medications the patient will have to use following surgery. Patient should have medical clearance from his or her primary care physician before undergoing cataract surgery to rule out any unknown co-morbid disease. CBC, chest xray, urinalysis, and a cardiac and respiratory function analysis Types of Acquired Cataracts o Nuclear Sclerosis Patient usually >60yo Increased optical density in the nuclear portion of the lens. Occurs secondary to oxidation, proteolysis of lens proteins. Brunescent color Due to amino acid residue Symptoms Slowly progressive visual loss or blurring over months to years o 1+ 20/20-40 o 2+ 20/40-80 o 3+ 20/80-200 o 4+ >20/200 Glare (#1) from ongoing headlights while driving at night o Loves sunny days and hates cloudy days, because pupils dilate and increase scatter. Image blur, and distortion also possible visual complaints Reduced color perception, especially reds and greens because blues and yellows are filtered. Retina may be indistinct with direct ophthalmoscopy Patients prescription shifts toward myopia= myopic shift, aka second sight. Patient sees better up close without their reading glasses Signs Slit lamp: yellow or brown discoloration of the central part of the lens o Brunescence means no change in VA. Sclerosis causes a change in VA. Distance VA decreases and near VA increases (PH increases DVA) Myopic shift in prescription Macular area normal Visual field: generalized degeneration DDx from those with increased pigment in lens, i.e. Af-Am Treatment If VA is decreased where prescription cannot improve it, and activities of daily living (ADLs) are affected. Consider referral for cataract surgery. o Cortical Cataracts o 27% In cortex Imbalance of electrolytes leads to overhydration of the lens. This leads to liquefaction of lens fibers, formation of vacuoles, and clefts. These lead to the separation of lamella. Spokes = water cleft Liquefaction = opaque Cataracts of the cortical region Several appearances o Small dots o Flecks o Vacuoles o Vossius Ring Symptoms Often asymptomatic until changes develop centrally May have some glare with headlights while driving at night. Signs Slit lamp: radial or spoke-like opacities in the lens periphery. They expand to involve the anterior and posterior part of the lens. VA distance- good until central involvement (no myopic shift) VA near- good until central involvement Visual fields: variable loss (usually none) Macula/ optic nerve are normal May be concurrent with nuclear sclerosis cataracts Seen better with DFE and direct retro illumination Treatment Same as with nuclear sclerosis Posterior Subcapsular Cataracts (PSC) 19% “Young Person’s Cataracts” Caused by loss of lens fiber nuclei, which are replaced by aberrantly migrating epithelial cells. Abnormal epithelial growth causes a “grainy” appearance due to abnormal cell migration. Side effect of steroid use and DM Looks like a spider web when viewed with a slit lamp. Results from cellular proliferation at the equator They migrate to the posterior pole This cataract results in the greatest loss of vision of any cataract, because it is nearest the nodal point of the lens. Symptoms Rapid loss of VA (weeks to months)- 5months o Because it is on the visual axis o Closest to the nodal point, so worst VA o Worse during the day (miosis) Decreased aVA at distance and near A lot of glare with headlights while driving at night o o o o o Signs Very hard to recognize familiar faces or signs Difficulty with reading is very common Cataract may be associated with: ocular inflammation (uveitis), prolonged steroid use, diabetes, trauma, radiation exposure. Classically occurs in younger patients (<50) If on axis, axis 20/100 (worse with PH). If off axis, 20/20 Bad because it is right at the nodal point Slit lamp: opacity appears near the posterior aspect of the lens. May or may not be along the visual axis. Forms a plaque. “Ground glass appearance” Best seen with direct retro illumination and optic section off the retina where it appears dark against the red fundus reflex Visual fields: variable Macular and optic nerve head are normal Treatment If on-axis: visually devastating requiring referral for cataract removal If off axis, need to monitor patient q6mo with education to watch q2-3 weeks to look for changes. Secondary cataracts are associated with ocular disease, systemic disease, or medications. Also trauma. Anterior Subcapsular Cortical on anterior below capsule Rare Is a side effect of anti-psychotic drug therapy Thorazine, phenylthiozine Proliferation of epithelial cells, which distorts optical wavelengths. Supranuclear Cataract Superficial to the nucleus Sutural Cataracts Supranuclear and cortical Opacities of the sutures Other less common cataracts Diabetic “Snowflake” Cataract Water imbibed in the lens Osmotic cataract Poorly controlled diabetes Cataractogenesis is the same for both DM I and II. DM must be present for 15-20 years before the cataracts develop, unless it is a very severe, uncontrolled case o But all still develop earlier than normal (~40yo) When the glucose concentration in the aqueous is high (>175mg/100 ml) the glucose concentration of the lens increases. o Results in an osmotic imbalance which attracts water Vacuoles form If the glucose concentration goes down, the vacuoles can disappear leaving “snowflake” opacities The excess glucose also binds to proteins (glycosylation) o Causes protein aggregation, cross-linking and insoluble proteins o Where glucose is decreased o In the Zone of Liquefaction o In cortex o Start of cataract formation The excess glucose can also be converted to sorbitol which can accumulate. Galactosemic Cataract Identical to DM Only difference is the origin of osmotic change In same zone and looks the same Problem with dulcitol No galactose is broken Decreased glutathione Hypoglycemic (Tetanic) Cataract Associated with hypocalcemia Associated with papilledema, diplopia, photophobia, and strabismus UV Induced Cataract Increased near the equator Causes vacuole formation Damages the lens, increases cataracts Wear sunglasses Radiation cataract o Causes increased membrane permeability o Problem with ion exchange and osmotic imbalance Glassblower’s Cataracts (Infrared Radiation) Also seen with welders Painful True exfoliation cataract Cataract rarely seen due to use of protective lenses Electric Cataract History of electrocution injury Anterior and posterior cortical changes Copper/Iron Cataract Intraocular foreign bodies containing iron and copper Copper produces- sunflower cataract Iron produces- brownish subcapsular cataract Christmas Tree Cataract Green and red areas with “balls” Due to myotonic dystrophy (i.e., Lou Gehrig’s Dx, MD) Traumatic Cataract If there is a cataract and the patient is less than 40yo, ask about trauma. Direct damage to lens fibers The more damage, the more the cataract. Appears wrinkled. Fully opaque. Vossius Ring o Blunt trauma o Iris hits the lens o No problem with VA Treatment Surgical procedures on the lens of the eye were first described around the 5th century in a technique called “couching.” This procedure was consisted of taking a sharp instrument and pushing the opaque lens out of the line of sight and into the vitreous. This technique increased the amount of light getting to the retina. Bach and Handel were rendered blind by the couching procedure at the hands of the same surgeon. In 1748, Jacques Daniel is credited with developing the first ECCE procedure. During WWII, Gordon Cleaver, a Royal Air Force Flight Lieutenant had been shot down. The impact blinded his right eye and left his left eye badly damaged from the cockpit windshield. It was Dr. Harold Ridley who observed the plastic material from the cockpit windshield in Cleaver’s left eye. But had not caused any further damage. Dr. Ridley went on to develop implantable lenses for cataract surergy. First surgery was done in 1949. It wasn’t until the 1970s when the IOL lens became acceptable and not referred to as a “foreign body.” Dr. Charles Kelman came up with ultrasound. This changed cataract surgery from a hospital stay to an outpatient procedure, from a very large incision to a small incision, and from a procedure that merely cleaned a clouded lens to a refractive procedure. Today there are over 1.8 million cataract surgeries performed each year. o Historical “Couching” Cataracts thought to be “corrupted humor” anterior to the lens. Anatomical recognition the lens itself at fault “couching” or pushing the lens back into the globe. o With finger to break zonules through the anterior chamber. In 1949, the first IOL implanted on a human was performed using a lens made from PMMA. In 1967, phacoemulsification and aspiration procedure In 1977, an IOL with flexible posterior haptics became available. 1980 brought the introduction of viscoelastic 1% sodium hyaluronate (Healon) which protected the corneal endothelium during IOL implantation. 1984- first foldable silicone IOL. Implant silicone foldable IOLs without need for cautery, scissors, or sutures. 1992- cataract surgery with only topical anesthesia. Improved with the addition of intracameral anesthesia. 1995- soft, foldable acrylic IOL received FDA approval for use in the US. o Early cataract surgery often produced large amounts of o As of 2007, more than 1.7 million procedures are performed annually. o Anesthesia First was cocaine Injected into the retrobulbar space causes anesthesia of the globe and muscles by blocking the branches of CN 3, 4, 5, and 6 (under the lower lid) Local anesthesia (sobconj injection) Advantages o Conducive to an outpatient surgery setting o o Quick recovery repair o Reduced incidence of nausea and vomiting Protocol used to achieve facial and retrobulbar block o Mild sedation (intravenously) o Orbicularis paralysis (facial nerve is blocked) o Retrobulbar block (to eliminate sensation) Complications of facial and retrobulbar block o Retrobulbar heme o Performation of the globe o ON damage o CRAO o CSN toxicity (CSF spread) Mre recently topical anesthesia is being used o To prevent risk of complications o Reduces patient stress and apprehension o Allows for immediate visual recovery o Pain is almost never a problem and eye movement is rarely a problem o Can easily convert to retrobulbar anesthesia ICCE and ECCE possible ICCE- Intra Capsular Cataract Extraction Take the capsule out via cryogenics. The entire lens is removes. Cryoextraction method 130-180 degrees corneoscleral incision at limbus. Creat iridotomy. Lens frozen to cryoprobe and delivered intact through corneoscleral incision Suture- creates astigmatism. Procedure can be traumatic. A large prescription is required after. This is an older method. Rare Uses anterior chamber and iris fixed lenses ECCE- Extra Capsular Cataract Extraction Leave the capsule in place. Anterior capsulotomy is performed and then the lens nucleus is expressed. A large incision (10mm) is needed. Aspiration-Irrigation Method Use surgical operation microscope Needle knife inserted through clear cornea into the lens Scalp-vein needle introduced into AC for irrigation Cortical and nuclear material aspirated through needle Single suture closure with intact posterior capsule ECCE- Phacoemulsification Method Anterior capsulotomy PE probe = ultrasonic vibration and aspiration and irrigation Nucleus and cortex fragmented and aspirated by ultrasonic probe 3mm scleral incision closed with single suture with posteruir capsule intact. o This is a refined version. The cataract is removed through a small (3mm) incision by breaking up the nucleus inside the eye, thereby eliminating the need for a large opening and decreased post-op cyl. Anterior capsulotomy is still performed. There is increased endothelial cell damage with the ultrasound. This is the most common method More recent techniques involve incision in peripheral cornea Eliminates bleeding and cautery Allows rapid surgery (15-20 min) Less painful (local antesthetic is adequate) More astigmatically stable if wound is <3.5mm Nearly eliminates risk of iris prolapsed and trauma Smooth, rapid, efficient phaco with minimal ultrasonic energy Whats Coming Soon Catarex- uses a high speed rotary propeller that creates a vortex and draws the cataract in Cataract liquefractire- uses hot BSS to liquefy the cataract and aspirates at the same time. Erbium YAG- laser utilizes to ablate the cataract Dodick photolysis- uses a laser to create shock waves to break down the cataract Photon Phacolysis system- similar to the dodick. Directs Nd:YAG at the nucleus phacoTmesis- similar to phaco. A 0 degree tip rotates at 4000 rpm. Intraocular Lens Implants (IOLs) Iris supported This is archaic. Square pupil Superior iris suture Sphincter erosion Irregular pupil Do not dilate these patients Anterior chamber No sphincter erosion, irregular pupil which can peak in direction of the ACIOL. This is the “back up” IOL if the capsule breaks and the posterior IOL cannot be implanted or intentionally sutured in place in the CB sulcus. Posterior chamber This is the most common Posterior radius varies according to the power. J loops vary with flexibility They fit in the bag Continuous curvilinear anterior capsulorhexis (1 continuous tear) so no loss of capsule. “In the bag” surgery CB erosion if out of the bag o IOL Materials PMMA o o CB sulcus position with flexible J loops Foldable PCIOL through 3mm PE incision 1st foldable = Mazzaco Taco Early IOLs were made from PMMA. Good IOL material. Can cause mechanical irritation and inflammation and it requires a larger incision for implantation. Excellent optical clarity and extremely biocompatible. Biocompatibility is essential to prevent inflammation and reduce deposits on the lens surface. Acrylic Further divided into hydrophilic and hydrophobic Soft, hydrophobic and mechanically weak. Copolymerization with methacrylates can maintain the flexibility of the acrylate and increase mechanical strength. Examples o Acrysof (Alcon) o Sensar (AMO) Acrylic Hydrogel Hydrophilic and can be copolymerized with a variety of monomers to produce desired properties. For IOL materials, acrylate and metacrylatebased materials are most commonly used with hydrogels. Silicone Medical grade silicone polymers have been used in drains in glaucoma surgery, scleral buckles and oculoplastic surgery as well as in IOLs. Associated with a higher incidence of PCO. Examples o RMX3 (Staar Surgical) o SLM2 (Allergan) About 25% of US ophthalmic surgeons prefer silicone foldable IOLs, but about twice this number prefer acrylic foldable IOLs. IOL Design IOLs are either one piece or three piece designs. One piece Plate haptic lenses are a form of one piece design. The optic is integral with the haptics Three piece The haptics are a different materials and are attached to the optic. These are more rigid Can be placed in the ciliary sulcus, a useful feature in patients with loss of capsular support. Edge Design Variations in the edge design of the lens can reduce the indicence of PCO. A squre edge on the posterior surface of the lens, which is in direct apposition to the posterior capsule of the lens, can act a barrier to the migration of residual lens epithelial cells responsible for PCO. In addition, careful design of the lens o o o o o edge can reduce the incidence of IOL related dysphotopsias by reducing unwanted reflections from the lens. Haptic Design Most haptics today are open loop design in a variety of styles. Also a variety of materials, including PMMA and polyporpolene or polyamide. Lens angulation, the planar relationship between the optic and haptic of an IOL, will determine the angulation of the lens as it positions itself in the capsular bag. A small degree of posterior vault of the optic will prevent papillary capture and help ensure contact with the posterior capsule. However, if the lens is inserted backward, it can result in a mild undesirable change in refractive outcome. Delivery System Each lens platform has a “delivery system” designed to implant the lens. variously known as “shooters” or injectors, these devices have become the most common way that IOLs are delivered into the eye following removal of a cataract. The smaller incision also allowed the incision to be “self sealing.” Lens delivery devices were developed to easily insert the lens into the eye in the folded configuration, control the placement of the haptics in the capsular bag, and allow the lens to unfold in a controlled fashion. Fabrication Current IOLs are either lathe cut or compression molded. Lens Platforms Major manufacturers have built a lone of products to support their lenses and this has become known as their “platforms.” Alcon lens platform is based on the AcrySof IQ lens design. The one piece design lens is made from a hydrophobic acrylic material is biconvex in shape, and is aspheric with negative SA of the cornea. The lens is compression molded and contains a blue light absorboing chromophore which absorbs light in the 400475nm wavelength range. AMO has developed a lens platform around the Technis lens. hydrophobic acrylic material and is available in both one piece and three piece designs. The lens is also available in silicone material. The lens is an aspheric biconvex lens with negative SA. The lens is lathe cut with open loop “C” shaped haptics made from PMMA. The edge is square at the posterior surface of the lens and is vaulted five degrees posteriorly. The lens has UV absorbers incoproated but no chromophores. B&L produces the Sofport lens platform. Three piece silicone lens. biconvex aspheric design and has no SA. The lens has a square edge with open loop “C” shaped haptics made form PMMA. The lens has a five degree posterior vault and “Violet Shield technology” They also have developed the Akreos lens platform. A one-piece hydrophilic acrylic material with an aspheric design. No spherical aberration. Lathe cut. Varying total diameters depending on lens power for improved centration. Posterior square edge and has a novel four haptic design for increased centration and stability. Other corrections Spectacles 20-30% image size o o Scotoma Contact lenses High plus (difficult to handle and see) Spherical IOLS Patient education Treats one focal point. As a result, you’ll have to provide the patient with spectacles or contact lenses post-op in order for them to achieve optimum vision. Full distance correction with reading glasses for near vision o This is what most patients will opt for. Full near vision correction with eyeglasses for distance vision Monovision o Successful monovision contact lens wearers make ideal candidates for monovision IOL implantation. Wavefront IOLs Research began in the late 1990s. first aspheric IOLs were introduced in 2004. Designed to decrease the amount of unwanted aberration that follows standard IOL surgery. The normal crystalline lens is initially oiblate in shape. This offsets the positive SA from the cornea. However, the crystalline lens changes shape during our lifetime and after about age 45, can start contributing additional positive SA to our optical system. When a traditional (nonaspheric) IOL is implanted, this causes a significant increase in positive SA to the eye. The young, healthy eye (birth to age 40) has a balance of positive spherical aberration (+0.25 microns) on the cornea with that of negative spherical aberration in the lens. as the eye ages, an increase in the magnitude of positive spherical aberration on the lens leaves the eye with unwanted spherical aberration. Positive spherical aberration is responsible for common night vision distortions, such as glare, halo, and poor contrast sensitivity. Wavefront IOLs have aspheric or modified prolate surfaces to minimize this distortion by better imitating the curvature of the cornea. Surgeons can measure a patient’s pre-op corneal spherical aberration and choose the aspheric IOL that will best help offset it. The goal is to leave the patient with about +0.10 microns which seems to produce the best contrast sensitivity and functional vision including for night driving. Surgery and incisions can induce HOAs such as coma. By measuring corneal SA preoperatively, the surgeon can select the appropriate IOL for the individual. Examples Technis Z9000(AMO) o A prolate surface that induces negative spherical aberration, thereby reducing or eliminating total spherical aberration. o Necessitates the correct centration and tilt of the Tecnis to avoid inducing new aberrations. o -0.27 microns spherical aberration o o AcrySof IQ (Alcon) o Back aspheric design o Intended to reduce the positive spherical aberration of the cornea. o -0.20 microns spherical aberration SofPort L161AO (B&L) o Aberration-neutral o Adds little to no SA to the eye, which maintains the overall positive SA of the mature eye. These need to be centered well to have the optimal effect. The higher the negative SA added, the more important centration becomes. Aspheric IOLs increase contrast sensitivity, and this will help your patients improve their functional vision. Wavlength Blockage All IOLs block some UV light, but blue-light absorbing IOLs are also available. Violet light is alos pote ntially phototoxic to the RPE. Blue light has less phototoxicity (longer wavelgnth and less energy), but its thought to be vital for scotopic and circadian photoreception, and it may attenuate vision and contrast sensitivity under low-illumination conditions and affect color perception. Blue-blocking lenses, which approximate the natural human crystallinelens with regard to light filtration are best. UV-blocking chromophores The normal crystalline lens typically filters blue light in the 200-400nm range. Studies show that blue light (400-500nm) can be harmful to the retina. Epidemiological studies suggest a correlation between excessive blut light exposure and ARMD. If we remove the crystalline lens and implant a lens that absorbs only UV light, the pseudophakic retina is at risk for phototoxicity due to exposure to blue wavelength of light. Physiologically, the RPE, as a result of the aging process, accumulates lipofuscin, which absorbs blue light, creating free radicals. These free radicals damage the RPE, resulting in geographic retinal atrophy. Added chromophores to the lens increase absorpotion of potentially harmful visible light in the violet and blue spectrum to simulate the characteristics of the natural lens. Examples AcrySof Natural (Alcon) o Blocks both UV and blue lights. Hoya AF-1 SofPort AO with Violet Shield Technology (B&L) o Filters a small amount of violet light but no blue light. Toric IOLs For patients with corneal astigmatism greater than 1.5D Toric lenses have etchings at 3 and 9 oclock positions. If the lens rotates greater than 20 degrees, immeduiately refer the patient back to the surgeon within the first month to preclude any further capsular fibrosis or contraction prior to the repositioning of the lens. Patient education o Going to recommend the lens power that is “closest” to correcting the astigmatism to give an excellent chance at optimum vision. Should there be any residual refractive error experiences, youre going to use the results of thepre-op corneal exam to select the best form of further refractive surgery There is a chance the lens can rotate. Any patient with more than 0.75D of corneal astigmatism will require some form of surgical intervention prior to IOL implantation. This could be a limbal relaxation incision, PRK, or LASIK. Types STAAR Toric (STAAR Surgical) o This lens is a plate haptic silicone lens with a 2D cylinder that corrects 1.4D at the corneal plane or a 3.5D cylinder that corrects 2.3D at the corneal plane. o Front surface toric o Single piece silicone IOL whose plate haptics have large holes to lock and stabilize the IOL in the capsular bag by migrating epithelial cells. Has a linear axis mark on the front surface’s periphery for axis alignment. o 2.00D and 3.50D, having effect of about 1.4D and 2.30Drespectively. o Silicone. One piece design. AcrySof IQ Toric (Alcon) o Available in cylinder powers of -1.50D, -2.25D, and -3.00D, giving 1.0, 1.5 and 2.0D cyl at the spectacle plane respectively. o The acrylic material has a “tacky” surface quality that promotes short-term stability and generates fibronectin and other natural tissue adhesives that stabilizes the implant to the capsule bag over the long term. o The haptics are open-loop modified L-haptics with three reference dost on each side that mark the axis of the cylinder on its posterior surface. Caveat: has the potential to rotate in the capsular bag after implantation. Patient may need to undergo a second surgery to rotate the lens back. For every three degrees of rotation, you lose approximately 10% of the astigmatic effect. Multifocal IOLs Four optical methods Zonal refraction o Oldest o Examples Iolab NuVue Central 2mm optic zone that provides approximately +2.50D of near add to the patient, while the remainder of the lens is for distance. AMO’s Array 5 zone zonal refractive lens AMO’s ReZoom AMOs 2nd generation multifocal IOL. FDA approved March 2005 Hydrophobic acrylic material Based on the Sensar AR40e platform 3-piece design, PMMA haptics o Provides continued centration critical to multifocal IOL performance. 6mm optic and blends 5 concentric zones throughout the entire optic portion of the lens, 13mm overall length Unique similarity to the Acuvue bifocal contact lens. Central, 3rd, and 5th zones are for distance vision while the 2nd and 4th zones provide near focus. o Aspheric transition between zones provides balanced, intermediate vision o Add power at the corneal plane is about +2.25D. Natural range of vision Caveat: may not be enough for some patients to see fine detail at near. If the pupil size is small (less than 2.5mm), since the center zone is solely for distance, there is good energy transfer of the distance image and virtually none for near energy. As the pupil enlarges near optical energy increases, and distance light energy diminishes. By the time the pupil reaches mesopic levels, the difference between near and distance optical energy creates a situation in which the two begin to interface with each other. Fair amount of optical disturbance. Patients report glare and halo problems, especially at night. OptiEdge Triple Edge Design o Patented design provides uninterrupted 360 degrees barrier protection and is designed to minimize edge glare. Studies o 92% of ReZoom patients “never” or only “occasionally” wear glasses. Diffraction o The diffractive zones all act together as a continuum. No one zone acts alone. As light diffracts from the diffractive edges, sporeading out in patterns from the edge junctions, the light from one junction begins to interact with light from many other junctions. Constructive interference and destructive interference both begin to occur. o Because all the diffractive rings act in synchrony, light from each zone goes to both the near and distance foci. The separation of these two foci determines the add power. o Optically there is at least an 18% visual loss to optical distortion, meaning that peak transmission of light is limited to 82%. This 18% of light does not simply dissipate, rather it readily creates optical disturbances, most noteable at mesopic light levels. o Examples Tecnis’ ZM001/ ZA9003 28 diffractive rings Not pupil dependent 5mm aperture Splits light energy to 41% near and 41% distance. The anterior surface is aspheric while the posterior has the diffractive surface. This surfaces ceates 4D of power, which is about 3.6D of effective add. Alcon Acrysof IQ SN60WF B&L SofPort AO Combination refraction/ diffraction o Apodized diffraction to optimize image quality in telescopic and microscopic optics. o Alcon ReStor FDA approved March 2005 6mm optical zone that is primarily distance oriented, apodization to create a diffractive central 3.6mm diffractive zone. The apodized diffractive zone is made up of a series of apodized concentric steps. Apodization, a gradual decrease in step heights from center to periphery, creates a smooth transition between the distance, intermediate, and near focal points. Meant to reduce the amount of perceived glare and haloes. 12 diffractive rings, with the innermost ring having a height of 1.3 microns, each step reduced from 3.6 by 0.2 microns. SN6AD1 A +3 add with a +2.50 at the spectacle plane. SN6AD3 A +4 add at lens plane equals +3.2 at spectacle plane. Allocates appropriate light energy according to activity and light levels. Minimize visual disturbances Acrysof platform Single piece UV-absorbing Acrylic Biomechanical advantages o Foldable implantation o Excellent centration o Capsular conformity Available in blue light-filtering chromophores Surgical procedure Same as with any oher Acrysof Must have perfect centration Single piece acrysof may not go in ciliary sulcus Slight hyperopic post-op result improves intermediate vision Has a yellow chromophore to filter both UV and short end blue light to protect the macula. Most haloes gone by 6-9 months (neuroadaptation) Difficult to remove because of haptics Works best at a plano to small amount of hyperopia. Caveat: patients with this lens may need to reduce their usual working distance to see clearly at near. Accommodative o Examples Crystalens (Eyeonics) Approved by FDA in Nov 2003. Responds to accommodative reflex. The IOL must move in the eye. Element of pseudoaccommodation. Range of distance, intermediate, and near vision. Patients perceive a “natural” transition of vision from far to near. The IOL incorporates a hinge into the plate design, allowing the optic portion of the lens to move in the eye in a way analogous to that of the natural crystalline lens. During near point activities, accommodation causes ciliary body contraction, the tension on the capsular bag decreases and ciliary body mass distribution forces vitreous displacement, allowing the optic to move forward. This provides near-point vision. Silicones Optics- 4.5mm square edge Hinges o Plate- 10.5mm Loop tip to loop tip- 11.5mm Monofocal optics Surgery o Mutliplane incision- any wound leakage can cause anterior vaulting. o Capsulorhexis larger than optic. o Injector may be used for insertion o Careful post-op evaluation. CCS (capsular contraction syndrome) Z syndrome Sometimes requires “accommodative rehabilitation” Works best for those that are slightly myopic. Less likely to produce glare, halos, or decreased contrast sensitivity. Some docs are mixing the types. Different in each eye. o ReSTOR and ReZoom are complimentary. o ReSTOR will do better for near in bright light conditions o ReZoom will do better for near in moderate to lower light conditions (restaurant lighting) This is generally reserved for the non-dominant eye. o Intermediate A few bilateral ReSTOR patients have intermediate vision problems. o Some surgeons are also performing modified monovision procedures, where one ye has a traditional aspheric IOL and the other eye has a multifocal IOL. Summary ReZoom ReSTOR Crystalens Distance Excellent Very good Excellent Intermediate Functional Good Very Good Near Very Good Excellent Poor Effective Add +2.6 +3.2 +1.5 Power Patient Selection Criteria Patients motivated to reduce their dependency on glasses Bilateral implantation candidates Cataract patients who did not need to wear glasses until presbyopia set in Patients who had been considering refractive surgery Patients with visual demands that are in normal light levels and normal reading materials Caution: Have unrealistic expectations Have a history of excessive complaints about glasses and contact lenses Drive at night as a profession Are happy wearing glasses Have pre-existing problems with nighttime glare Have pre-existing, significant ocular surface disease o Education Methods and Topics Each patient should be fully informed of the risks, benefits and alternatives to surgical cataract extraction. Cataract is simply causing less than perfect vision, but that it is not damaging the eye or affecting the fellow eye. Deferring surgery will not increase the risk of surgical complications or make the procedure more difficult unless the cataract is very advanced. Provide all prospective cataract surgery patients with a packet of information explaining cataract surgery and introducing advanced lifestyle IOL options at the time of cataract diagnosis. Post-Op o Meds Cyclosporine Combined with the hourly use of artificial tears for the first few days and post-op tapered qid for the 1st 4-6 weeks has provided many ocular surface patients with a few added lines of vision on the Snellen acuity chart. Antibiotics Vigamox 0.5% to prevent infection Tid x1 week Generally discontinued after week 1. Steroidal anti-inflammatory Prednisolone acetate 1% to control anterior chamber inflammation secondary to surgery. Tid x1 week, then bid x4 weeks Tapered over one month Non-Steroidal Anti-Inflammatory Drugss (NSAIDs) Nevanac 0.1% to control CMEand to provide additional inflammation control. Tid x1 week, then bid x4 weeks. Tapered over one month Steroids and NSAID can be extended to 6 weeks for elective IOL patients. o Schedule varies from doctor to doctor 1 day Subjective assessment (pain, redness, and vision) Remove shield and protective eye patch Clean wound site VA at distance for standard and toric IOLs VA at distance, intermediate, and near for Multifocal IOLs. o If you demonstrate inproved near VA at this early stage, it builds excitement and anticipation for the second eye surgery. Capitalize on the results patients are sharing with you. SLE with applanation tonometry o o o o o Cornea The cornea should be clear. Pay special attention to the presence of microcystic edema, as this is almost always an indicator of increased IOP. If IOP is high, manage with drops and/or oral agents in the usual manner, and discharge the patient when stable. If patients present with significant stromal edema and Descemet’s folds, increase the frequency as every one-two hours whiule awake depending upon severity to facilitate visual recovery. Anterior Chamber The Ac should be relatively quiet. It is not unusual to observe rare cells. Significant cells and flare in the absence of corneal edema should be managed by increased topical steroid frequency. If the anterior chamber reaction is severe as if looking into a snowstorm and is associated with central corneal edema, a relatively quiet eye (no significant injection), consider toxic anterior segment syndrome (TASS). The surgeon should be notified and a retinal consultation may be indicated. Tear Film Manage tear film insufficiencies aggressively. Preservative free tears, long-lasting nonpreserved artificial tears, ophthalmic gels, prescription agents such as Restasis and punctual plugs. Check position of the IOL Check condition of the posterior capsule for opacification DO For Toric IOLs o Dilate the operated eye to ensure proper axis alignment. Three dots will be found at the periphery of the optic 180 degrees apart from one another. The alignment of the peripheral dots on the optic should match the planned corrected axis of astigmatism. A good indication of whether the axis if properly aligned is uncorrected VA. If the VA is found to be within acceptable range (>20/30) at this visit, that is strong empirical evidence that alignment is achieved. Management plan o Use shield or glasses during the day for protection/ shield at night o Steroid/ antibiotic gtt q4h o Analgesics prn o Restrict physical activity o RTC 1 week 1 week Same as work-up as 1 day post-op o Offer assurance VA should be crisp and measured in the same fashion as day one. SLE is performed in the same manner as day one. Any corneal edema or folds noted at day one should be cleared at this visit and the AC should be deep and quiet. If corneal edema and folds persist and/or an AC reaction persists, I recommend performing gonioscopy to search for a retained lens material in the angle. Retained lens material in the eye can induce chronic inflammation and may be cause for patients to return to the OR for irrigation. If the angle is clear, I recommend dilating the eye to search for retained lens material. Most instances of retained lens fragments are benign, and theremnants will slowly resorb with time. With any retained lens material finding, consult the surgeon. Management plan o D/c antibiotics o Reduce steroid drops to bid o RTC in 2-3 weeks 2-4 weeks Same entrance tests as one week visit DFE, especially if BVA is less than 20/100 o At this point, during the post-operative experience that CME may manifest. Be alerted to this entity with any drop in BCVA. A cystic appearance to the macula will be evident and the diagnosis can be solidified with OCT. Management plan o Taper gtt (tid, bid, qd x1 week) o RTC x3weeks 6-8 weeks Same entrance tests as one week visit Keratometry/ Refraction (final SRx given) DFE (if not done on previous visits) Management plan o d/c shield and drops o resume normal physical activities o return in 2 weeks if final SRx not given yet 6 months Common issues dealth with at this visit are generally qualitative issues such as haloes and glare, fluctuating vision, blurry vision, and missed expectations. Etiology for these issues are quickly discovered after a careful clinical examination. They are typically related to PCO, TF abnormalities, or residual refractive error. 1 year Education Educate the patient about what the procedure involves, how long it will take, what post-op medicvations will be required and when the patient can safely return to work. o Review the importance of continued care and warning signs of complications. Any change in vision, a sudden increase in floaters, flashes of light, eye pain and red eye. Tell the patient to call the office immediately if any of these occur. Medications given Broad spectrum antibiotic to prevent infection A potent steroid (usually Pred Acetate 1%) to decrease inflammation NSAID to decrease the likelihood of CME. All applied frequently (up to 6x a day) for the first week and gradually tapered over the next 3 weeks. Sunglasses since some patients are more sensitive to light after surgery. Complications Complications of Retrobulbar Anesthesia Possibility of reacting CNS Close to the ON with superior gaze (ON drops) Vulnerable structures include the ON, long posterior ciliary artery, short posterior ciliary artery, vortwx veins, short ciliary veins Requires a lot of patient control o They can be sedated Tear Film Abnormality Eyelids- Ecchymosis Conjunctiva Conjunctival injection Subconjunctival hemorrhage Chemosis Extra Ocular Muscles Acquired diplopia o Due to speculum restricting EOMs. This resolves with time. Operative Wound High corneal astigmatism (mainly with planned ECCE) Wound leakage Persistent foreign body sensation Glare Unwanted images after cataract surgery, known as dysphotopsias, generally occur in about 1 in 10 cataract surgeroes from a variety of causes. However, only 1 in 1,000 requires a lens exchange. Tx options o Lens replaced (lens explantation) with another model o Mild miotic (0.5% pilocarpine or Alphagan) Lens Dislocation with Pseudoexfoliation o Starts with the subjective complaint that the lens was moving somewhat with eye movements (pseudophacodonesis) o PXS is the most common cause of IOL dislocation. o At risk for late dislocation, as the contraction of the anterior vcapsule opening is more extensive in eyes with PXS. o Cornea Other reasons include capsule shrinkage for any reason and zonule damage from any cause, including damage during surgery. Dislocation without Pseudoexfoliation o Reasons: trauma, inflammation, zonular damage at the time of cataract surgery and progressive zonular damage related to capsule contraction forces. o Some IOL lens designs may be more likely to cause capsule contraction than others. Greatest with plate haptic silicone IOLs and least with acrylic hydrophobic IOLs. o Tx: Complete vitrectomy followed by removal of the IOL, followed by insertion of an anterior chamber IOL o The most common cause of IOL decentration occurs when a surgeon inserts the inferior haptic into the capsular bag and the superior haptic into the ciliary sulcus, which causes upward movement of the IOL optic. Superior migration of PCIOLs produces the “sunriswe syndrome” while inferior migration produces “sunset syndrome.” o Papillary capture is when some or all of the optic is within the pupil and the rest of the lens is behind the iris. It iusually occurs 1-2 months post-op, and the incidence with silicon eor acrylic foldable lenses is very low. Phacoemulsification can cause dislocated crystalline lens fragments into the vitreous cavity. Surgeons can reduce the chance of this happening by using the lowest possible vacuum and power settings and infusion pressures. Epithelial basement membrane changes Epithelial abrasions Superficial punctuate keratitis Corneal edema o Secondary to munor trauma to corneal endothelium during surgery. o Treat with steroids and/or topical hyperosmotics o Due to decreased endothelial cells o Normal: 3200/mm2 o A 65 yo has about 1800. o Limit is 700-800 o If there is a known Fuch’s Endothelial Dystrophy, surgeons may elect to do a “Triple Procedure” which is ECCE, IOL, and Penetrating Keratoplasty Descemet’s MembraneDetachment (rare) Endothelial deposits Bullous Keratopathy (L) o Very common o Due to compromised endothelial function due to endothelial loss. o Greater incidence with ACIOLs and iris-fixated IOLs, but less than 1% with ECCE and PCIOLs. o Clinical processes Vertical stria (fluid in stroma) Stromal folds (thickening and opacification) Formation of microcysts Formation of bullae o Management Hypertonic saline drops or ointment PKP Intraocular Pressure Transient rise in IOP o Usually related to inflammatory response in the anterior chamber. o Other possible causes Steroid responder This occurs in about 1/3 of patient who use the medications qid for 4-6 weeks. Blood in anterior chamber (hyphema) Retained cortical material and lenticular debris Tightly sutured wound o Tx Reduce the steroid drops or add Timoptic, or both. Topical prostaglandins are contraindicated, because of their potential association with CME. Pupillary Block Glaucoma o Secondary to direct blockage of pupil by IOL optic or vitreous prolapsed. This causes an increase in the pressure in the posterior chamber if the aqueous cannot leave the posterior chamber where it is produced. This results in a forward movement of the peripheral iris, which eventually occludes the TM. o Seen more often with ACIOL and ICCE All anterior chamber lenses, in the absence of an intact capsule, must have additional iris incisions (iridectomy) to prevent this. o Treatment by dilation of the pupil and with subsequent PI Can reposition the footplate as well. Hypotony (low IOP) o Usually secondary to wound leaks o Other conditions that can create hypotony Inadvertent filtering bleb Cyclodialysis cleft Ciliary shutdown secondary to severe inflammation Choroidal detachment Retinal detachment Anterior Chamber Iris Anterior chamber inflammation and persistent iritis in patients after ECCE and PCIOL implantation probably averages about 2%. It results from the breakdown of the blood-aqueous barrier. Uveitis-glaucoma-hypehma (UGH) more commonly occurs with anterior chamber IOLs, but it occasionally occurs with PCIOLs. It is a rare complication and results from mechanical trauma on the angle or iris. Papillary block glaucoma results when the IOL blocks aqueous flow through the pupil. Malignant glaucoma occurs when anterior aqueous flow is misdirected posteriorly. Hyphema o If mild May resolve on its own in a few days to several weeks Steroid drops Consider cycloplegic o If severe Steroid drops Antifibrinolytic agents Anterior chamber washout Bedrest Shallow Anterior Chamber o Indicative of wound leak or papillary block glaucoma Iris atrophy Traumatic mydriasis Papillary distortion o ACIOL- caused by haptic o PCIOL- due to fibrosis of tissue o Vitreous or iris incarceration into the wound Vitreous Wick Syndrome High risk of CME and RD with vitreous loss Rebound Iritis (L) Endophthalmitis An intraocular bacterial infection that usually occurs within the first two or three days after surgery. Common organisms o Gram + Staph. Epidermidis (40%) Staph aureus (20%) Strep species (10%) o Gram – Pseudomonas and Haemophilis (15%) o Fungi, proprionobacterium, misc (15%) Often source of infection is not known but possible causes include o Normal bacterial flora of the conjunctiva o Nonsterile instruments o Contaminated solutions o Contaminated IOLs Symptoms o Moderate to severe ocular pain and rapid loss of vision Clinical signs o Lid swelling o Bulbar conjunctival injection o Corneal edema (haze and/or folds) o Severe anterior chamber reaction and hypopyon o Vitritis with poor or no view of the fundus The presence of a filtering bleb poses a heightened risk for infection over a patient’s entire lifetime. Within a 5-year period after cataract surgery, 23% of patients may develop a bleb-related complication that could lead to endophthalmitis. When combating endophthamitis, mere hours can make a significant difference in overall visual outcome. Late infection rates of 2-3% have been reported with blebs located superiorly; even higher rates have been associated with inferiorly-placed blebs. Pay careful attention to the bleb, and monitor the patient for decreasing vision, anterior chamber cells, pain and redness. If a glaucoma patient has a trabeculectomy and presents with a red eye, you must consider the possibility of endophthalmitis. Monitor daily. Secondary Cataracts/ Posterior Capsular Opacification (L) This is the most common. It occurs in up to 50% of ECCE procedures, usually from 6 months to 5 years. Missed anterior lens epithelial cells replicate along the posterior capsule. Seen after ECCE IOL surgery. The crystalline lens’ epithelium exists on the surface of the anterior lens capsule (where A-cells are located) and on the surface of the capsule at the equator (where E-cells are located). Anterior supcapsular opacities with some aphakic PCIOLs result from A-cell proliferation. E-cells are primarily responsible for “secondary cataracts.” o Anterior capsule opacification (ACO) usually occurs with a month or so after cataract surgery. o PCO clinically manifests as fibrous membrane and Elschnig Pearls. Typically, the fibrous opacities develops 2-6 months post-op and is more common with silicone IOLs, while the Elsching pearls can develop several months to years after surgery and are more common with PMMA IOLs. The rates are believed to be lower with acrylic IOLs. Aka Elsching Pearls Best viewed with retroillumination o Undilated or dilated with DO or slit lamp Symptoms include gradual VA loss and occasionally glare or monocular ghost images Tx o Intervene early with earliest signs of PCO to address the patient’s complaint. o Neodymium: YAG laser post-capsulotomy Windshield Wiper Syndrome Uveitis-Glaucoma-Hyphema (UGH) Syndrome Due to an ACIOL that is poorly designed and therefore causing irritation of the iris and angle with resultant chronic iritis, recurrent hyphemias, and secondary glaucoma. Cystoid Macular Edema (CME)- occurs mostly with DM This is the second most common complication of modern cataract surgery, after PCO, and is the most common cause of visual decline during the iommediate post-op period after uncomplicated cataract surgery. More common with ICCE than with ECCE o ECCE incidence of angiographic evidence is about 15%, but clinically symptomatic CME is only about 2%. Typically appears 1-3 months after surgery, usually in the first 6 weeks, but infrequently can occur months or years later. Usually it resolves spontaneously, but about 1% of cases are chronic and last more than 6 months. Use of ketorolac tromethamine both pre and post-op significantly decreases both angiographic and symptomatic vision problems of CME. Due to either increased permeability of perifoveal capillaries secondary to the release of prostaglandins or mechanical traction from the vitreous. Occurs as high as 50% of cataract surgeries but only persists in 2-6% Definitive diagnosis made with FA, with its characteristic early leakage and late staining petaloid pattern Treat with oral and topical NSAIDs (Acular) and/ or steroids (Pred Forte) o For 1-3 months followed by slow tapering. o If topical is not effective, then a sub-Tenons or intravitreal steroid injection if the fallback treatment. No one really knows why CME occurs. One theory: CME occurs when prostaglandins are released as the result of surgical trauma. CME may also occur more often if the posterior capsule is ruptured or if there was much inflammation during the surgery. CME usually presents 3-6 weeks after cataract surgery, with a peak incidence at 6 weeks. Visually significant in 1-6/9% of patients. Any patient with CME should be evaluated by a cataract surgeon. The intact lens capsule serves as a barrier between the anterior and posterior chambers. When the capsule is broken during surgery, the incidence of posterior complications increases. A greater incidence of both RD and CME has been reported in all references to complications of cataract surgery. Reoeated effect oif trauma stimulates enough prostaglandin release to cause a small area of CME. The denser the nucleus, the more time and instrument energy are needed to phacoemulsify it. Increased time inside the eye and additional instrument energy both may contribute to a heightened risk for complications. May also experience a longer recovery time due to associated corneal folds, corneal edema and endothelial striae. Earlier referral would make nucleus phacoemulsification less of a risk, because less energy would be required. RD Most occur within the first three years following surgery The phacoemulsification instrumentation system that is used to break up the eye’s crystalline lens requires energy, which is then transmitted to the vitreous and may accelerate the clumping and shrinking of that structure. A vitreous detachment follows, with all the risks of associated break formation. If the liquefied vitreous gets under the break before the RPE cells can seal it, an RD will take place. When a full-thickness break takes place, the retinal pigment cells are dislodged and may be seen in the anterior vitreous (Schafer’s sign) Like CME, see more in ICCE than in ECCE due to loss of the capsular barrier and anterior shifting of vitreous with resultant vitreoretinal traction With modern surgery, though, the posterior capsule acts as a barrier to help prevent forward shifting of the vitreous. Moderate to high myopes are most at risk after cataract surgery. The younger patient (<60yo) who has not had a posterior vitreous detachment has some increased risk. Surgical trauma also a risk Complaints of seeing a few more floaters are common after surgery. This is likely due to improved vision from surgery. Surgery accelerates vitreous syneresis. Posterior capsultomy has a 4x higher risk of developing RD, probably because of loss of hyaluronic acid from vitreous, its destabilization and disruptions in the vitreoretinal interface. Surgeons may reduce the incidence of PCO by intraoperative polishing of the posterior capsule and/or present day acrylic or silicone IOLs. RD after ECCE has a less than 1% incidence, much less than after ICCE. RDs usually occur within 6 mnths post-op. Major risk factors for post-op RD are pre-op conditions such as high myopia (8x higher risk), retinal lattice degeneration (10x higher), family history of RD and hereditary vitreoretinal degeneration. Intraoperative factors that predispose to RD include crystalline lens fragments, disruption of the posterior capsule (13x higher risk) and Nd:YAG laser capsulotomy (4x higher risk). Usually occurs within the first few months after surgery. More common in younger individuals who are moderately to significantly myopic. A dramatic increase in pigment in the vitreous sugfgests a retinal break caused by vitreous traction. The additional pigment comes from the retinal pigment epithelium, not the iris. If the lens nucleus is dropped, you need to see a retinal surgeon to make sure that an autoimmune reaction does not occur. Residual Refractive Error Unplanned residual refractive error can be managed with laser vision correction, piggyback IOLs, spectacle or CL use. Do not hesitate to bring the surgeon back into the management of an unhappy patient. Reading difficulty o Can do refractive surgery after if the patient is unhappy with refraction. Examples o Pt 1, 70yo Hx: Pseudoexfoliation This is a fairly common disorder, affecting 4-6% of patients past the age of 60 and increases significantly with age. The condition weakens the lens zonules, predisposing the patient to additional zonule problems during phacoemulsification nd is the most common cause of IOL dislocation. Usually unilateral. Is associated with an increased incidence of cataracts Forms dense deposits on both the anterior and posterior lens capsule, resulting in capsular opacification and subsequent fibrosis. The firbosis, coupled with the PSX, results in contraction of the entire capsule and breaking of the weakened zonules. Dislocation of the intact capsule with the IOL inside takes placeeven if there is no preexisting zonule weakness from PSX. Associated with poor pupil dilation Not always associated with glaucoma Follow patients with PXS closely for development of glaucoma.