Isosteric heat of hydrogen adsorption on MOFs
advertisement
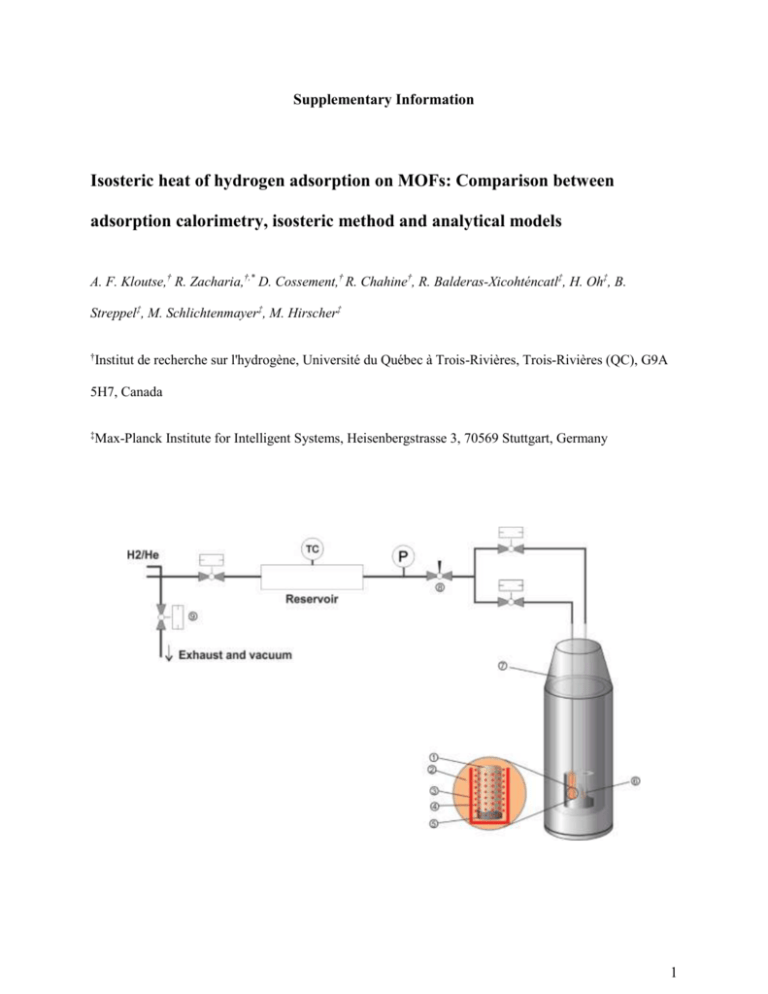
Supplementary Information Isosteric heat of hydrogen adsorption on MOFs: Comparison between adsorption calorimetry, isosteric method and analytical models A. F. Kloutse,† R. Zacharia,†,* D. Cossement,† R. Chahine†, R. Balderas-Xicohténcatl‡, H. Oh‡, B. Streppel‡, M. Schlichtenmayer‡, M. Hirscher‡ † Institut de recherche sur l'hydrogène, Université du Québec à Trois-Rivières, Trois-Rivières (QC), G9A 5H7, Canada ‡ Max-Planck Institute for Intelligent Systems, Heisenbergstrasse 3, 70569 Stuttgart, Germany 1 Figure S1. Schematic representation of the coupled volumetric-calorimetric setup. 1. sample cell, 2. calorimetric well, 3. heater, 4. thermopile, 5. sample, 6. reference cell, 7. Tian-Calvet calorimeter, 8. needle valve, 9. pneumatically actuated ball valves. Adsorption Calorimetry Simultaneous calorimetric-manometric measurements were performed using an experimental setup that couples a commercial Tian-Calvet type micro-calorimeter BT 2.15 (SETARAM) with a home-built Sievert’s volumetric adsorption measurement system; a schematic of which is shown in the Figure 2. The interior of the calorimeter consists of an insulated calorimetric block with two identical calorimetric wells referred to as reference chamber and sample chamber. The heat flux measurement zone of both calorimetric wells features a 3D Calvet heat fluxmeter sensor which is essentially a thermopile consisting of an axial array of 9 cylindrically arranged concentric rings containing a total of 684 thermocouples (sensitivity = 30 µV/mW, resolution = 0.10 µW). The measurement zone accommodates two high pressure stainless steel cells (rated for 100 bar and 300 °C), one for samples and other used a reference. Cryogenic measurements were carried out by coupling the calorimeter to liquid nitrogen cooling system (LNCS) while a small flow of helium is maintained through the thermopiles which allows good thermal contact between the sample cell, reference cell and thermopile. The simultaneous measurement of adsorption and heats were achieved by attaching the sample cell and reference cells to the Sievert’s volumetric measurement system by means of pneumatically actuated ball valves and a needle valve. The volumetric measurement system consists of a gas dosing manifold which is provided with a calibrated digital pressure transducer (Dresser DXD, Heise, range = 0–100 bar accuracy = 0.02 % at full scale) and a type K thermocouple (Omega, accuracy = ± 0.5 °C) for measuring pressure and temperature of the dosing and equilibrated gases. The temperature of 2 manometric system installed within an isothermal box was controlled within ± 0.3 °C. The control of valves and acquisition of pressure and temperature were carried out using National Instruments FieldPoint controller and modules which is interfaced to a standard PC using NI LabVIEW™ Professional Edition, while the acquisition of heat flow signals and the control of liquid nitrogen flow into the calorimeter were made possible using the Calisto Software™ (SETARAM). Calibration. (1) Calibration of calorimeter. Calorimeter was calibrated using both Joule-effect method and reference material method. Joule-effect method is an absolute calibration which makes use of high efficiency of Calvet calorimeter (up to 95 % of the heat produced is transmitted to the sensor) and uses no metallic reference standard material. In the Joule-effect method, the cells are heated by passing known dc currents (I) through a platinum resistance electrical resistance (R) merged onto the thermopile of reference cell. The heat generated is directly obtained from Joule’s first law, Q = I2R. In the second calibration method which uses a reference material method, the temperature sensors and energy scale of the calorimeter were calibrated in the standard differential scanning calorimetry mode using the extrapolated onset of indium melting (429.75 K) and heat of fusion of indium (28.45 J K-1) performed at a heating rate of 1 K min-1. Accuracy of calibrations was verified by comparing measured specific heat capacities of pure synthetic sapphire (standard reference material 720, NIST) and copper from a pristine high purity oxygen-free high conductivity copper gasket used for ultra-high vacuum cryogenic applications with that of report data.34,35 Average relative deviation of our measured heat capacities from the reported reference data is ~0.5% for sapphire and ~3% for copper. (2) Calibration of reference, sample cell volume and cold volume of Sievert’s system. Calibration of empty volumes of reference and sample cells were performed by expanding known helium gas 3 (99.999%, Praxair Inc.) from the dosing volume to cells. This method based on the conservation of mass involves two expansions: (1) expansion of helium at initial pressure and temperature in the dosing manifold to an empty sample cell having final equilibrium pressure and temperature and (2) expansion of helium from the dosing manifold to the sample cell containing a known volume of non-adsorbing material, such as quartz or stainless steel beads. The volumes were obtained by solving mass conservation equations where the real gas densities were determined from the thermodynamic equation of state of real gases provided by NIST REFPROP Standard Reference Database. The cold volume of the system was measured by immersing previously expanded helium in the calorimetric cells into cryogenic tank at a pre-determined height. The calculation of cold volume involves solving the mass balance for helium before and after immersion. (3). Dead volume of MOFs. To measure the dead volume of MOFs samples, accurately weighed samples (0.1 – 0.2 g) were loaded into the sample cell. The sample transfer was done hermetically within an argon-filled glove box workstation to safeguard moisture and air sensitive MOFs. Filled sample cell and the empty reference cell, latter required to stabilize the heat flow signal are inserted into the calorimeter wells and argon gas is expelled. Prior to measurements, the MOFs samples are outgassed by heating to 125 ºC and evacuating for 24 hours. The dead volume of the samples was measured by expanding helium from dosing volume to the sample cell. For these measurements, the final temperature and pressure of helium gas were respectively restricted to 298 K and less than 2 MPa so as to minimize any possible helium adsorption by MOFs. Due to large volumes of system, we did not observe any influence of valve volumes on the final equilibrium pressures. 4 Figure S2. Spurious heat measured by dosing hydrogen into the blank calorimetric cells maintained at 87, 97, 100, 113 and 117 K correlates linearly with incremental pressure. The linear regression by fitting these data is used to calculate the spurious heat contributions during adsorption tests. 5 Figure S3. Representative excess adsorption of hydrogen on MOF-5, Cu-BTC, Fe-BTC, MOF177 and MIL53 measured at least two different temperatures using adsorption calorimetry. Also shown are the excess adsorption isotherms of hydrogen on MOF-5 measured at 87 K36 and 100 K.14 The maximum uncertainty in our excess measurements is ± 3% of the reported excess. 𝐻𝐹 = 𝐻𝐹0 + 𝐴 ∙ 𝑒𝑥𝑝( −𝑡−𝑡0 ) 𝜏 , [3] where, HF0 is the baseline value of heat flow rate at equilibrium and τ is a characteristic time constant. For Cu-BTC we obtained τMOF = 256.4 ± 2.2 s, τblank = 254.7 ± 2.6 s, where the standard deviation is estimated for different incremental dosing at 77 K. This similar relaxation time scale is rather expected because of thermal design aspect of calorimetric system with small sized samples embedded inside a large heat conducting metal block.27 Isosteric Method 6 Adsorption isotherms: An automated Sievert’s type apparatus (Setaram-HyEnergy PCTPro2000) was used with a so-called micro-doser (MD) from HyEnergy designed to measure the storage properties accurately with only small amount of sample (~100 mg). The PCTPro-2000 with a micro-doser is capable of performing PCT measurements with the hydrogen gas at a maximum pressure of 25 bar. A general illustration of this volumetric apparatus (PCTPro-2000) with the main components is shown in Figure S4. The original setup was coupled with an in-house made cooling-heating device regulated by a temperature controller. This configuration allows us to perform hydrogen adsorption measurements in a wide temperature range (77 K to 300 K). Figure S4a shows the schematic illustration of PCTPro2000 setup. For isotherms at 77 K and 87 K, the sample holder is submerged in liquid nitrogen or argon, respectively (Fig. S4a). For temperatures above 87 K, the sample container is enclosed by a copper block (orange) with a cooling finger submerged in liquid nitrogen (Fig. S4b). The liquid nitrogen level is controlled within 0.2 cm height by an automatic liquid N2 refilling system. The copper block includes a K-type thermocouple and a heater connected to a PID unit control. The experimental setup is capable to maintain a constant temperature within ±1 K over the whole temperature range. 7 Figure S4. Schematic diagram of the Sieverts´ apparatus coupled with a cooling system (77 K300 K) a) Dipping method with dewar cryostat for cooling at liquid nitrogen (77K) and liquid argon (87 K) temperature. b) Cooling system for temperatures above 87 K where the sample container is enclosed by a copper block (orange) and partially sank in the liquid nitrogen. The liquid nitrogen level is constantly controlled by automatic liquid N2 refilling system. 8 Volume calibration: The sample container volume (Vcon) is defined as volume of the empty sample holder minus the skeletal volume of the sample (Vske) and can be directly measured by a helium expansion test at room temperature. The helium expansion test consists in the measure of the pressure change when we let expand a known volume of helium given by reservoir volume (Vres) into the sample container. However, the helium expansion test at low temperatures results in an inaccurate value of Vcon for microporous materials due to a significant helium adsorption. In order to subtract the contribution of the non-adsorbed hydrogen in the sample container volume (Vcon), we measure the hydrogen uptake for a non-adsorbing reference material (sea sand) for different Vske and temperatures. Using the reference material uptake we can calculate the so-called ‘excess amount of hydrogen’, nexcess(p,T), which is given by nexcess(p,T) = nexperiment(p,T) –nsea sand (p,T) where nexperiment(p,T) is the raw data of hydrogen uptake measurement for the porous sample (total amount of hydrogen present in the sample container) and nsea sand (p,T) is the sea sand calibration for a given temperature and Vske. The Figure S5 shows an example of the application of volume calibration corrections to hydrogen adsorption data (in wt% uptake) for the material MIL-53 at 77 K. It shows that the calibration corrections at low temperatures can be significant even at pressures below 20 bar. 9 Excess hydrogen uptake / wt% 5 4 3 2 1 RAW Excess H2 Uptaken 0 0 5 10 15 20 P / bar Figure S5 Hydrogen adsorption by MIL-53 at 77 K exhibiting the raw data (nexperiment(p,T)) without cold volume correction (Red) and excess hydrogen uptake (nexcess(p,T)) corrected for cold volume (Blue). 10 Calculation of absolute adsorption: The absolute adsorption is calculated from the excess adsorption with approximated excess volume of the adsorbed layer and adsorbed gas density. We assume that the adsorbed layer density is equal to the hydrogen liquid density (ρl = 0.07 g ml-1), and then the excess volume, Vexcess, of the adsorbed layer can be approximated as 𝑉𝑒𝑥𝑐𝑒𝑠𝑠 = 𝑛𝑒𝑥𝑐𝑒𝑠𝑠 · 𝑀𝐻2 𝜌𝑙 In addition, we should also consider the amount of gas that would be present in the pores due to the external pressure in the absence of adsorbent-adsorbate interaction. This can be calculated from the corrected ideal gas equation 𝑛𝑔𝑎𝑠 = 𝑃 ∙ 𝑉𝑒𝑥𝑐𝑒𝑠𝑠 𝑍·𝑅 ∙ 𝑇 with the correction (compression) factor37 for non-ideal gas Z given by Z = (1.000547- (6.07•10-7)T+(0.000912-(1.0653•10-6)T)•P+((7.373407-0.0901T)•10-7)•P2) where pressure, P, is given in atm, T is temperature and R is a gas constant. The absolute adsorbed amount, nabsolute, is therefore: 𝑛𝑎𝑏𝑠𝑜𝑙𝑢𝑡𝑒 = 𝑛𝑔𝑎𝑠 + 𝑛𝑒𝑥𝑐𝑒𝑠𝑠 = 𝑛𝑒𝑥𝑐𝑒𝑠𝑠 · (1 + 𝑃 · 𝑀𝐻2 ) 𝑍 · 𝜌𝑙𝑞 · 𝑅 ∙ 𝑇 11 MOF Adsorption Isotherms: Data obtained for Cu-BTC, MOF-5, MOF-177, MIL-53 and FeBTC-Xerogel adsorption isotherms. The MIL-53 heat of adsorption was calculated based on the isotherm experiments for the temperatures 77 K and 87 K. Figure S6: Excess hydrogen adsorption (full symbols) and desorption (open symbols) isotherms of CuBTC ( 77 K, 87 K, 93 K, 128 K, 158 K, 218 K, 296 K) 12 Figure S7: Excess hydrogen adsorption (full symbols) and desorption (open symbols) isotherms of MOF5 ( 77 K, 87 K, 97 K, 107 K, 117 K, 296 K) Figure S8: Excess hydrogen adsorption (full symbols) and desorption (open symbols) isotherms of MOF177 ( 77 K, 87 K, 97 K, 107 K, 117 K, 296 K) 13 Excess hydrogen uptake / wt% 2 1 0 0 5 10 15 20 P / bar Figure S9: Excess hydrogen adsorption (full symbols) and desorption (open symbols) isotherms of MIL77 K, 87 K, Excess hydrogen uptake / wt% 53 ( 296 K) 2 1 0 0 5 10 15 20 25 P / bar Figure S10: Excess hydrogen adsorption (full symbols) and desorption (open symbols) isotherms of FeBTC-Xerogel ( 77 K, 83 K 87 K, 91 K, 100 K, 110 K, 125 K) 14 15