Summary
advertisement

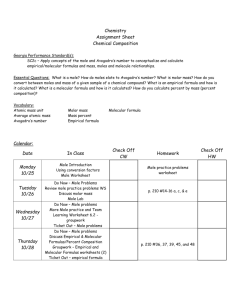
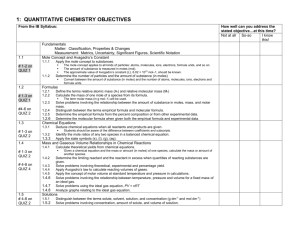
Monday Content Standard: SC2c: Apply concepts of the mole and Avogadro’s number to conceptualize and calculate Empirical/molecular formulas Mass, moles, and molecules relationships Molar volume of gases Essential Question: How is the mole, an unfamiliar unit, related to more familiar units like grams, particles, and liters? How is dimensional analysis used to make conversions between all of these units? Musical Selection: Mayer Monday Learning Activities: [ 25 minutes ] Percent Composition Warm-Up – Review of Chem-To-Go Lesson 25 Video [SC2c) Summary: Students watched a video lesson over the weekend to learn about percent composition calculations. Each student will be given an index card containing a % composition question as he/she enters the classroom. They will find a seat with a randomly selected small group of peers. They’ll calculate to answer their own question and trade with three other students to complete a total of four % composition problems in 15 minutes. Teacher will visit each group to gather informal diagnostic data for use in tomorrow’s flexible grouping. Teacher will answer lingering questions. [ 60 minutes ] Tiered with Student Choice - Mole Calculations Stations: Foundational to Advanced [SC2c] Summary: Students will be asked to visit a foundational, an intermediate, and an advanced mole calculations station. As students are early in the learning process with this difficult content, students are invited to choose a combination of seven questions to answer during the activity. A few rules are in place to prevent students from choosing only foundational-level questions: Foundational: Students should choose at least one but no more than three questions. Intermediate: Students should solve at least one question. Advanced: Students should solve at least one question. [ 5 minutes ] End of Class Announcements [SC2c] Summary: Teacher will collect tiered activity work, remind students to watch the lesson 26 video, and encourage students to come prepared for an intense activity tomorrow! Differentiation Plan: Modified activity for student needs: Provided three levels of difficulty and invite students to choose based on their individual needs at least one of each level and any additional four questions to solve during a 60 minute learning station activity. Informal assessment of progress: Collect informal data via observation of each student during the warm-up activity. Store data on blank class roster for use in tomorrow’s flexible grouping. Assessment Plan: Collection of informal diagnostic data: Collect informal data via observation of each student during the warm-up activity. Store data on blank class roster for use in tomorrow’s flexible grouping. Formative marking and data collection: Collect student’s tiered activity work at the end of class, mark mistakes, return to students, and encourage them to re-visit questions/answers in small group help. Homework: Watch Chem-To-Go Lesson 26: Empirical and Molecular Formulas, complete Cornell notes, and watch post-video quiz. Tuesday Content Standard: SC2c: Apply concepts of the mole and Avogadro’s number to conceptualize and calculate Empirical/molecular formulas Mass, moles, and molecules relationships Molar volume of gases Essential Question: How are percent composition and mole conversions used together in the real-world to identify chemical compound samples? Musical Theme: Jamie Cullum Learning Activities: [ 15 minutes] Empirical Formula Warm-Up – Review of Chem-To-Go Lesson 26 Video [SC2c] Summary: Students watched a video lesson last night to learn how to determine empirical and molecular formulas of unknown compounds. Each student will be given an index card containing an empirical formula question as he/she enters the classroom. They will find a seat with a small group of peers. Each student will solve his/her own empirical formula calculation and confer with peers if needed. Teacher will answer lingering questions. [ 65 minutes ] Interactive Mole Airlines: Identifying Chemical Samples [SC2c] Summary: The activity takes place in three parts: part one = analysis of a sample, part two = identification of victims, part three = comparison of class data and discuss of scientific method in real-life. Part One – Analysis Stations: Students analyze data collected on chemical samples collected from fictional bodies at a fictional plane crash site. Mass spectrometer graphs and percent composition data are given for each unknown sample. Students will work in a small group [homogeneous: based on yesterday’s performance and last night’s quiz data] to calculate the empirical formula from the unknown sample and compare their conclusions to a given list of known compounds. Part Two – Identification Discussion Groups: Students will jig-saw into six new groups. Each group should contain at least one member from each of the six analysis stations. Members will share analysis data to compile an identification list of 12 unknown samples collected from fictional victims. Members will apply the chemical analysis and identifications to victim diagrams and passenger biographies to identify the eight fictional victims. Part Three – Collection/Comparison of Class Data: Identification groups will write their identification opinions on the Smart board, and students will discuss the process in terms of scientific method. [ 10 minutes ] Summary of Empirical Formulas [SC2c] Summary: The concept of empirical formulas is abstract, and the mole airlines activity is meant to give students a more concrete perspective on the content. Students will discuss and answer 4 questions to help in processing the activity and mastering the concept. Finally, students are asked to solve one empirical formula question on their own before class tomorrow. Differentiation Plan: Flexible grouping using diagnostic data: Students are grouped in the lab analysis homogeneously according to performances in yesterday’s warm-up and last night’s video quiz. More complex samples are given to students who show stronger skills in this area. Differentiation of content to encourage critical thinking at the appropriate level of challenge: Students in higher performing analysis groups are given samples that are more complex to calculate. Students in lower performing analysis groups are given samples that require fewer steps to calculate. High learning expectations for all: Every chemical sample is beyond the level of our standards. Every student is working at college-level. Assessment Plan: Use diagnostic data to create flexible groups: Students are grouped in the lab analysis homogeneously according to performances in yesterday’s warm-up and last night’s video quiz. Informal assessment of student learning: Teacher visits each station during the analysis phase to observe calculations. Struggling students are noted on a blank class roster to be helped tomorrow during class time. Homework: Review Lesson 26 video if needed. Solve empirical formula homework question. Prepare for tomorrow’s mole conversions quiz. Wednesday Content Standard: SC2c: Apply concepts of the mole and Avogadro’s number to conceptualize and calculate Empirical/molecular formulas Mass, moles, and molecules relationships Molar volume of gases Essential Question: How are percent composition and mole conversions used together in the real-world to identify chemical compound samples? Musical Selection: The Middle Learning Activities: [ 40 minutes] Mole Conversions Quiz [SC2c] Summary: Students perform conversions of mass, moles, molecules, and molar volume using dimensional analysis. [50 minutes] Empirical and Molecular Formulas Think Tac Toe [SC2c] Summary: Students are asked to choose at least three of nine problems to solve individually. They are asked to “win think tac toe” with their choices in a horizontal, vertical, or diagonal line. Problems are arranged so that every student will solve an easy, intermediate, and advanced question. An incentive of one bonus point is given to encourage students who would like to solve only the three most challenging problems. Differentiation Plan: Differentiation of level of difficult by self-evaluation: Students will select at least three of nine problems to solve. All students are encouraged to consider solving the three most challenging problems by offering a bonus point incentive. Assessment Plan: Formal assessment of Mole Conversion Quiz: Teacher will grade, record, and return the quiz. Use quiz data to inform tomorrow’s activity and grouping: Students will be grouped homogeneously according to needs diagnosed on the quiz. Additional practice, small group tutoring, and appropriate challenge will be provided in tomorrow’s critical thinking activity. Common, appropriate assessment: Today’s Mole Conversions Quiz was developed collaboratively with other honors chemistry teacher. Homework: Review any videos. Solve practice packet examples. Prepare for tomorrow’s empirical and molecular formula quiz. Thursday Content Standard: SC2c: Apply concepts of the mole and Avogadro’s number to conceptualize and calculate Empirical/molecular formulas Mass, moles, and molecules relationships Molar volume of gases Essential Question: Have I mastered the skills and standards of the unit? Am I thinking critically enough about the mole concept to be successful on the summative assessments? Musical Selection: Relaxation Station Learning Activities: [30 minutes] Empirical and Molecular Formulas Quiz [SC2c] Summary: Students will determine the empirical and molecular formulas of compounds by applying percent composition and mole conversion skills. [50 minutes] Critical Thinking Review - Tiered Problem-based Learning: How can sugar be converted to oxygen gas? [SC2c] - Tiered Mole Conversion Riddles [SC2c] Summary: Students will be assigned to a lab station with other students who are performing similarly. Each group will solve a problem-based learning situation. Three tiers of the assignment exist, and advanced learners will be challenged with an AP-level application. After solving the PBL, students will shift to solving mole conversion riddles. The problems are also tiered so that lower performing students are solving slightly more straightforward questions. [10 minutes] Review for Cumulative Quiz and Lab Practical [SC2c] Summary: Teacher will remind students of possible content on tomorrow’s cumulative quiz by reviewing a chemical reaction and net ionic equation. Students are also encouraged to view the PBL video on Edmodo. Differentiation Plan: Use diagnostic data to create flexible groups: Students are grouped in the critical thinking activity homogeneously according to performances in yesterday’s quiz. Differentiation of content to encourage critical thinking at the appropriate level of challenge: Students in higher performing groups are given more complex problems to solve. Students in lower performing analysis groups are given more straight-forward problems to solve. Assessment Plan: Formal assessment of Empirical and Molecular Formula Quiz: Teacher will grade, record, and return the quiz. Common, appropriate assessment: Today’s quiz was developed collaboratively with other honors chemistry teacher. Homework: Review any videos. View PBL video in Edmodo. Prepare for lab practical and cumulative quiz. Friday Content Standard: SC2c: Apply concepts of the mole and Avogadro’s number to conceptualize and calculate Empirical/molecular formulas Mass, moles, and molecules relationships Molar volume of gases Essential Question: What are the expected products of the chemical reaction? What indicators of a reaction are observed? Musical Theme: Ben Folds Five Learning Activities: [ 15 minutes] Cumulative Quiz Summary: Students will demonstrate mastery of unit 5 skills by answering two short answer questions. [ 75 minutes ] Lab Practical Summary: Students will individually visit a lab station to solve an advanced mole conversion problem. Differentiation Plan: Flexible grouping using diagnostic data: Students will enter the lab practical according to their recent performances on quizzes and labs. Stronger students will be invited to go first, and others will have time to review prior to the practical. Assessment Plan: Common, Appropriate Formal Assessment (SC2a, b, and c): Lab practical and cumulative quiz developed with other HHS chemistry teachers for use in honors chemistry classes only. Homework: Review any videos. Solve practice packet examples. Prepare for Monday’s test.