New Assign Sheet Mole
advertisement

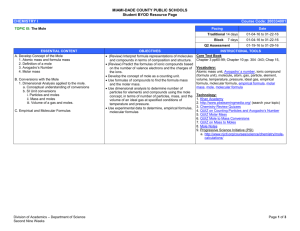
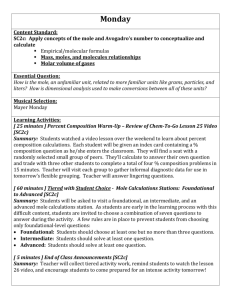
Chemistry Assignment Sheet Chemical Composition Georgia Performance Standard(s): SC2c – Apply concepts of the mole and Avogadro’s number to conceptualize and calculate empirical/molecular formulas and mass, moles and molecule relationships. Essential Questions: What is a mole? How do moles elate to Avogadro’s number? What is molar mass? How do you convert between moles and mass of a given sample of a chemical compound? What is an empirical formula and how is it calculated? What is a molecular formula and how is it calculated? How do you calculate percent by mass (percent composition)? Vocabulary: Atomic mass unit Average atomic mass Avogadro’s number Molar mass Mass percent Empirical formula Molecular formula Calendar: Date Monday 10/25 Tuesday 10/26 Wednesday 10/27 Thursday 10/28 In Class Mole Introduction Using conversion factors Mole Worksheet Do Now – Mole Problems Review mole practice problems WS Discuss molar mass Mole Lab Do Now – Mole problems More Mole practice and Team Learning Worksheet 6.2 groupwork Ticket Out – Mole problems Do Now – Mole problems Discuss Empirical & Molecular Formulas/Percent Composition Groupwork – Empirical and Molecular Formulas worksheets (2) Ticket Out – empirical formula Check Off CW Homework Mole practice problems worksheet p. 210 #14-16 a, c, & e p. 210 #36, 37, 39, 45, and 48 Check Off HW Friday 10/29 Do Now – Molecular Formulas & Percent Composition problems Review Empirical and Molecular Formulas worksheet Mole Quiz Monday 11/1 Do Now – Mole Problems Lab: Percent Sugar in Gum Lab: Calculating the Percentage Composition of M & M’s Practicing Moles/Quick √ Percent Composition Practice Problems from Chapter 6 Tuesday 11/2 Election Day Holiday Wednesday 11/3 Practice Problems 6.1, 6.2,and 6.3 Thursday 11/4 Review for Unit Test Mole Review Worksheet Friday 11/5 Test Chemical Composition – Mole Read science article and answer comprehension questions Chemistry Take Home Mole Quiz Ch. 6 Supplemental Problems