What is the purpose of this experiment
advertisement

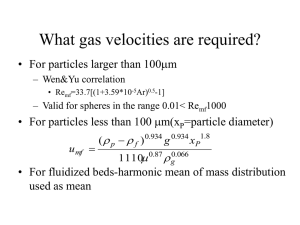
Fluidized Bed Reactor University of Illinois Fluidized Bed Reactor Lab Prep Report Unit Operations Lab 2 March 02, 2011 Group 6 Liliana Gutierrez Linda Quan Lipi Vahanwala Priya Chetty Sana Buch Vijeta Patel Unit Operations ChE-382 Group No. 6 p. 1 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois 1. Introduction A fluidized bed reactor is defined as a reactor in which gases or liquids flow through an unconstrained bed of particles at a certain fluid velocity to cause the particles to move in a fluid-like manner. These units are much more complex than fixed packed beds and require a much larger vessel than a packed bed reactor. In addition, a fluidized bed reactor requires a lot of pumping power depending on the size and density of the solid material. Furthermore, when using fine particles, the particles themselves can easily be entrained in the fluid or gas and carried out of the reactor; therefore, a separation unit to extract the particles is required. In addition, these fine particles can cause erosion and damage to internal components of a system which can be expensive and difficult to control (Perry). On the other hand, fluidized beds provide a greater heat transfer rate between the bed and the shell of the unit due to the rapid mixing motion. The concentration of the fluidized bed is homogeneous which can be good for separation, and the reactor can be operated at a continuous state. Fluidized bed reactors can be used for both catalytic and non-catalytic processes. Some catalytic processes include the oxidation of naphthalene to phthalic anhydride, the production of polyethylene and ammoxidation of propylene to acrylonitrile. Some noncatalytic uses of fluidized beds include roasting of sulfide ores, coking of petroleum residues, calcinations of ores, combustion of coal, and the incineration of sewage sludge (Perry). These systems can also be used for heat transfer between gases and solids, temperature control, solid mixing, gas mixing, drying of solids and gases, adsorption, desorption, heat treatment, and coating. In this experiment, a vertical cylindrical column is used as the platform for the experiment and the set up is similar to Fig. 1.1. Unit Operations ChE-382 Group No. 6 p. 2 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois The column will be filled with various grain sizes and air will be used as the gas which will flow into the column from the bottom. The grain sizes, air flow rate, pressure drop, and the bed depth will be recorded and used to determine the minimum fluidization which is the point at which the particles are suspended. As the flow rate of the gas through the particles increase, the drag force on the particles increase. When this drag force is equal to the weight of the particles in the bed, the particles become fully suspended in the column. The particles are then known to be “fluidized”. If the gas flow rate is further increased, bubbles will begin to form and the particle behavior begins to resemble that of fluids (Perry). The experiment will be conducted again under the same conditions, but at different temperatures to observe the effects of temperature on the minimum fluidization. 2. Literature Review/Theory In a fluidized bed, the packing is supported by the up-flowing phases and thus behaves much like a liquid. This packing phase is in constant motion within the contactor. In the fluidized bed: Unit Operations ChE-382 Group No. 6 p. 3 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois (1) The rapid mixing motion in the bed gives a high heat transfer rate between the bed and the shell of the unit; thus, heat can easily transfer towards or away from the bed. (2) The bed unit tends to be quite uniform in concentration when compared to the nonmixed packed bed. This can be an advantage or disadvantage for a given separation or chemical reaction. (3) The packing can recycle. In other words, the packing can flow out of the unit for separate treatments and back into the unit. The main objective of this experiment is to measure the hydrodynamics such as, pressure drop of the fluidized bed by performing one phase experiment using air as the gas. The calculated data will then be used to compare with existing correlations. The Ergun equation can be used to describe the pressure drop of the bed at low gas velocities. However, as the flow-rate increases the pressure drop becomes the constant and does not change with increasing gas flow-rate. This point is defined as fluidization. As the gas flow-rate increases further, violent mixing within the bed begins to occur. This causes the formation of large gas bubbles passing through the bed. If the gas flow-rate is decreased, the graph of pressure drop verses the flow-rate does not exactly follow the curve. This is due to significant hysteresis effect due to the frictional forces in the initial bed. Unit Operations ChE-382 Group No. 6 p. 4 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois Figure 2.1: relationship of superficial velocities with pressure drop and bed height (Subramanian) Figure 2.1 describes the behavior of the bed particles as the upward superficial fluid velocity is gradually increased from zero to the point of fluidization and back down to zero. In the initial state of the fluidized bed, there is an initial gas flow-rate in which the pressure drop is zero and the bed has a certain height. As the superficial velocity increases (path ABCD with arrows going to the right), the pressure drop gradually increases while the bed height remains constant. This is the region where the Ergun equation for the packed bed can be used to relate the pressure drop to the velocity. In the Figure 2.1 when the point B is reached, the bed begins to increase in height while the pressure drop levels off and ceases to increase. At this point, the upward force exerted by the fluid on the particle is sufficient to balance the net weight of the bed and the particles begin to float in the fluid (fluid here is understood to be the gas or air in this case). As the velocity of the gas is increased further, the bed continues to increase in the height, but the pressure drop is constant. As the superficial velocity starts to decrease (in the reverse direction), the behavior of the bed particle follows the curve DCE. The pressure drop stays constant while the bed settles back down. When point C is reached, the pressure drop begins to decrease. The bed height becomes the constant while the pressure drop follows the curve CEO. Point C in Figure 2.1 is the minimum fluidization velocity, V. After the point of fluidization, the bed particles begin to settle back into a loosely packed state. Therefore, the constant bed height in the reverse direction is larger than the bed height in the forward direction. The fluid mechanics of fluidized beds are very complex. Therefore, semi-empirical correlations for the minimum velocity of fluidization have been developed based on the particles’ Reynolds number. The basis of these correlations is that the minimum fluidization velocity for the uniform particles can be predicted by using the force balance. Therefore, at minimum fluidization, the drag force extorted on the bed of particles by the fluid is equal to the force of gravity on the bed. The upward force on the fluidized bed can be described as: And the volume of the particle can be described as: (1-ϵ)*AL Unit Operations ChE-382 Group No. 6 p. 5 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois Hence, the net gravitational force on the particle can be described as (Subramanian): (1ϵ)*AL * (ρs – ρg) g This yields the following equation: Where, A = bed cross section area (ft^2) ΔP= pressure drop across the bed (lbf/ft^2) V= bed volume at minimum fluidization (ft^3) = bed void fraction at minimum fluidization (no unit) = density of the solid (lbm/ft^3) = density of the gas (lbm/ft^3) When the Reynolds number, Re, is low and Péclet number, Pe, is high, both inertial and thermal elects are negligible. Furthermore, the hydrodynamic interactions lead to highly complex behaviors that have defied explanation. For the particles with small diameters (D≤0.1mm), the Reynolds number is very small (Re≤ 10) which requires the use of the Kozney-Carmen Equation. It is important to note that a small Reynolds number for the fluidized bed implies that there is a steady parabolic flow across the thickness of the bed, with the velocity vanishing on the walls of the cell (Tee). For this experiment, air flow-rate and pressure drop across the bed will be measured in order to obtain the Reynolds number. The Reynolds number can be measured using the equation below: In order to correlate this Reynolds number with the Ergun Equation, the following equation has been derived by Wen and Yu that will be used for this experiment = (33.72 – 0.0408Ar) 1/2 – 33.7 Where, Unit Operations ChE-382 Group No. 6 p. 6 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois Ar= Archimedes number= µ= viscosity of the fluid ) = diameter of the particle (ft) = minimum fluidization velocity (ft/s) The pressure obtained from this equation may be compared to the experimental pressured drop. 3. Experimental 3.1 Apparatus Unit Operations ChE-382 Group No. 6 p. 7 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois 14 13 4 5 6 15 12 3 16 11 7 10 2 1 9 8 Figure 1. The Fluidized Bed apparatus Following are the major parts of the apparatus: Component Description Unit Operations ChE-382 Group No. 6 p. 8 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Purpose Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois 1 ½ “ Smith Ball valve Control air flow rate into the sand column 2 ½ “ Crane Co. Globe valve Control air flow rate into the Gilmont flow meter 3 4 0-100 psig Omega Engineering, Inc pressure Monitor the pressure of the air entering gauge into the sand column 0-100 % Gilmont flow meter Monitor the flow rate of air into the sand column 5 Plexiglas column with I.D. of 9.8cm Houses the sand fluidized bed reactor 6 0-50 in H2O Monometer Monitor the pressure drop across the fluidized beds in both columns 7 Powerstat variable transformer Control the temperature of the silica fluidized bed 8 Fluke 2166A Digital Thermometer Monitor the temperature of the silica fluidized bed 9 ½ “ Smith Ball valve Control air flow rate into the silica column 10 ½ “ Crane Co. Globe valve Control air flow rate into the F & P Co. flow meter 11 Type K thermocouple Used in conjunction with the Fluke digital thermometer to monitor the silica fluidized bed 12 Plexiglas column with I.D. of 9.8cm Houses the silica fluidized bed reactor 13 ½ “ Wilkerson air inlet pressure regulator Maintains a constant inlet pressure of air into the system 14 0-200 psig Wilkerson pressure gauge Monitor air inlet pressure going through the air inlet pressure regulator 15 0-100 % F & P Co. flow meter Monitor the flow rate of air into the silica column 16 ½ “ Ball valve Control the inlet of external air to the system Unit Operations ChE-382 Group No. 6 p. 9 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois 3.2 Materials and Supplies Following is the list of materials and supplies used in the experiment: 1 Component Description Purpose Ottawa Standard Sand 20-30 mesh Used in the column for the one of the fluidized beds 2 Silica Used in the column for the one of the fluidized beds 3 (2) 250 ml Erlenmeyer flasks Prevent sand from entering the manometer 4 (2) Rubber stoppers with holes Allow tygon tubing to the Erlenmeyer flasks and then connection to the manometer 5 Tygon tubing Connect the column, Erlenmeyer flask, and manometer to each other 6 Vacuum Cleaning out the column and spills on the floor 7 100 ml Graduated Cylinder Accurately measure the void fraction of the sand and silica 8 9 USA standard testing sieve ranging 3360- Get a consistent fluidized bed by filtering out 25 μm the larger products Ruler Measure the height of the fluidized bed during each flow rate 10 Micro Pipette bulbs Create a seal on the column walls 11 External air supply Used throughout the system for data analysis 3.3 Experimental Procedure 1. Determine the average particle size as follows: A. Arrange several sieve trays with the largest micron value at the top and the smallest at the bottom. Unit Operations ChE-382 Group No. 6 p. 10 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois B. Place packing material into the top sieve tray and allow it to travel through the sieve trays by shaking. C. Weigh and record the mass of material in each sieve tray. 2. Determine the Packing material void fraction as follows: A. Place small amount of dry particles in a graduated cylinder and record the apparent volume. B. Measure out 50.0 ml of water in another graduated cylinder. C. Slowly add dry particles to the water and wait until the particles settle. D. Record the volume of combined water and dry particles. E. Obtain the void fraction of packing material. F. Load the fluidized bed with fine silica up to 6 inches. G. Allow all particles to settle down by gently tapping the fluidized bed column. H. Turn on the main air supply. I. Slowly turn on the air flow by carefully opening valve corresponding to the silica fluidized bed. J. When operating with one fluidized bed, make sure the air flow to the other fluidized bed is completely closed. K. Increase the air flow rate by 5-10% and with each increment record the gas flow rate, pressure drop, height of the bed that the silica or sand occupies, and visual changes that occur in the fluidized bed. L. Do not decrease the air flow rate until minimum fluidization is reached. M. After the minimum fluidization is reached, begin to decrease air flow rate by 5-10% and with each increment record the same characteristics as in step K. N. Do not increase the air flow rate until the zero velocity is reached. O. Increase silica temperature by using Powerstat variable transformer. P. Repeat steps G through I. Q. Load the second fluidized bed with Ottawa sand up to 7 inches. R. Repeat steps G through I for the second fluidized bed. S. Load the fluidized bed with Ottawa sand up to 5 inches. T. Repeat steps G through I. U. Shut down system by turning off air supply and closing all valves. Unit Operations ChE-382 Group No. 6 p. 11 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois V. Vacuum sand particles in the two columns and the area of experiment. 4. Anticipated Results In the fluidized bed reactor experiment, the behavior of the fluidized bed is examined by varying the type of particles utilized and finding the minimum fluidization velocity. Furthermore, the reactor bed is fluidized when it starts to bubble and maintains bubbling without losing the sand or silica grains. Graphical analysis can be used to find a correlation between air flow rate, pressure drop, and the minimum fluidization velocity. Graphically, as the pressure profile decreases, the slope increases and the minimum fluidization velocity can be determined. Additionally, the Ergun equation can be used to determine the theoretical minimum fluidization velocity when the particle’s bulk density and quality of the air are specified. Theoretically fluidizing the bed particles and allowing the particles to return to their stationary phase creates looser packing between the particles. This leads to an increase in void fraction and an increase in bed height. Tighter packed bed results in an increased friction factor because the bed impedes the air flow throughout the bed while looser packed beds with larger void fractions decrease the friction factor. Consequently, superficial air flow velocity increases as the friction factor decreases. The Reynolds number in a fluidized bed is independent of the height and temperature of the bed. Only the void fraction and the diameter of the particles are used to determine the Reynolds number. Finally, the heat exchanger in the silica bed generates heated air to enter the packed bed column resulting in heat exchange between the silica grains. Consequently, as the superficial velocity of the air increases, the pressure drop in the bed increases resulting in the particle suspension at the minimum fluidization velocity. Past the minimum fluidization velocity point, any increase in gas velocity results in turbulent bubbling. 5. References 1) Perry, R. H., and D. W. Green. Perry's Chemical Engineers' Handbook. New York: McGraw-Hill, 2008. Print. 2) Subramanian, R. Shankar. "Flow through Packed Beds and Fluidized Beds." Clarkson University. Web. 1 Mar. 2011. Unit Operations ChE-382 Group No. 6 p. 12 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois <http://web2.clarkson.edu/projects/subramanian/ch301/notes/packfluidbed.pdf> . 3) Tee, Shang-You, P. J. Mucha, M. P. Brenner, and D. A. Weitz. "Velocity fluctuations in a Low-Reynolds-number fluidized Bed." J. Fluid Mech 596 (2008): 467-75. Cambridge University Press, 18 Oct. 2007. Web. 1 Mar. 2011. <http://www.amath.unc.edu/Faculty/mucha/Reprints/JFMfluidized.pdf>. Unit Operations ChE-382 Group No. 6 p. 13 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois 6. Appendix I: Job Safety Analysis (formerly called WP &C) What is the purpose of this experiment? The purpose of this lab is to increase air flow incrementally through a bed of fine particles of Ottawa sand, sea sand, and silica with varying dimensions until the bed is fluidized. The volumetric air flow-rate will be regulated and the pressure drops will be recorded. There is a heater to heat the silica bed from ambient temperatures while the sand bed will be maintained at ambient temperature. What are the hazards associated with the experiment? The sand and silica in the columns are known to spill onto the floor around the column under careless operation. The floor around the unit is slippery as a result. Constant maintenance of the laboratory area must be conducted to prevent slipping and falling while walking near the unit. Compressed air is used to fluidize the bed. Care must be taken to wear eye protection so that suspended particles do not blow into the eyes of the operator. The heater is located directly adjacent to the fluidized bed lab apparatus which could expel excessive amounts of heat which can cause burns. Silica is hazardous if inhaled and can cause damage to the eyes, skin, and digestive tract. How will the experiment be conducted in a safe manner? The floor needs to be kept clean by using the vacuum cleaner to remove particles that spill onto the floor. Eyewear must be worn at all times to protect the eyes from sand or silica, which can be expelled from the column or through leaks in the column by the compressed air. Individuals handling the silica should be sure to wash their hands prior to eating or any other activity. Care should be taken not to inhale silica dust particles. What safety controls are in place? The air inlet regulator has an internal diaphragm to maintain a constant inlet pressure of 40 psig. If the pressure surges, the regulator valve will relieve the pressure to maintain the inlet pressure. The vacuum cleaner has a filter adapter attached to it in order to prevent excessive dust accumulation when cleaning up the lab area and adjusting the height level of the bed in the column. Unit Operations ChE-382 Group No. 6 p. 14 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016 Fluidized Bed Reactor University of Illinois Describe safe and unsafe ranges of operations. Operating at a superficial velocity below the settling velocity will prevent blowing sand or silica out the top of the column. Controlling the increments of increasing air flowing through the column will also prevent the discharge of the bed out the top of the column. The silica column cannot be heated to temperatures greater than 110°F. Plexiglas melts at is approximately 265 ° and operating conditions should never reach these temperature ranges. I have read relevant background material for the Unit Operations Laboratory entitled: “Fluidized Bed Reactors” and understand the hazards associated with conducting this experiment. I have planned out my experimental work in accordance to standards and acceptable safety practices and will conduct all of my experimental work in a careful and safe manner. I will also be aware of my surroundings, my group members, and other lab students, and will look out for their safety as well. Signatures: _Sana Buch_____________________________________ _Priya Chetty____________________________________ _Liliana Gutierrez_________________________________ _Vijeta Patel_____________________________________ _Linda Quan_____________________________________ _Lipi Vahnwala__________________________________ Unit Operations ChE-382 Group No. 6 p. 15 Buch, Chetty, Gutierrez, Patel, Quan, Vahanwala Spring 2011 2/8/2016