program - Retina International
advertisement

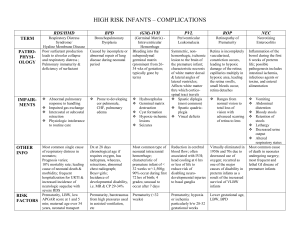
On World Retina Day 2015 Retina International Calls on Members to Pledge Support for the Development of Patient Reported Outcomes. Millions of adults and children across all continents will mark World Retina on Saturday September 19, 2015 by pledging to support Retina International in the formulation of agreed patient reported outcomes to aid the development of clinical trials in Retinal Diseases. Advances in the understanding and treatment of retinal degenerative diseases, both rare and inherited and age related, will significantly reduce the emotional, social and economic burden of sight loss. Patients now have the opportunity to participate in a meaningful way in the development of life changing therapies that will benefit them and future generations of their families. Before a therapeutic intervention in any disease can be approved for use, it must undergo extensive testing in large, multi-centre clinical trials to demonstrate efficacy. Approval is contingent on the successful achievement of endpoints in clinical trials and the selection of these is critical to the development of new therapeutic agents. An endpoint is a measurement by which a trial is deemed as success or a failure. Although developing standards for clinical trials in ophthalmology sets a basis for meeting adequate safety and efficacy outcomes, the evolution of medicine requires that these standards be continually reviewed and updated. The involvement of patients affected by retinal disorders in the development of agreed endpoints is well supported by researchers, clinicians and regulators. Retina International seeks to play an increasing role in defining what is globally-relevant in terms of how we measure symptom impact and functionality, how we define disability and describe adverse events, what is treatment tolerability? and what improves quality of life?. The importance of defining patient-relevant outcomes in clinical trials for evaluating ophthalmic medical drugs and devices cannot be understated. Participating in evaluation processes is a responsibility Retina International enthusiastically accepts and seeks to promote. On World Retina Day, Retina International, an umbrella group of over 50 patient-led Vision organisations in 33 countries across all continents is calling on its members to pledge support and play their part in expediting this critical process. “Retina International encourages patients to take every opportunity to inform clinicians and researchers of the relevance of any clinical measures – it is the patients, and only the patients, who can adequately describe the total impact of a treatment in a visual world and functionality therein” said Christina Fasser – President of Retina International. She added “Patients are becoming increasingly educated in the development of their own healthcare. They understand their voice must be heard if future generations are to benefit from treatments that deliver meaningful outcomes leading to greater independence, quality of life and the ability to fully participate in society” Ends For further information contact Christina Fasser at Retina International Phone +41 (0)44 444 10 77 Fax +41 (0)44 444 10 70E-mail: cfasser@e-link.ch www.retina.ch www.retina-international.org