L - Zn_HCl labEDITED.12
advertisement

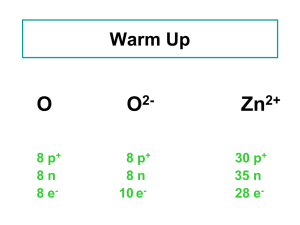
An Application of the Laws of Conservation of Matter and Definite Proportions Chem. 319 Ms. Hobbie Lots of changes from what I used… the original 2012 version is too confusing. Background The origins of chemistry as a science can be traced back to the origins of the atomic theory of matter. The person who first put together a coherent atomic theory to help explain chemical processes (i.e. reactions) was John Dalton, an English schoolteacher/chemist, who in 1808 formulated the atomic theory of matter. Dalton developed this theory based on the work of scientists who came before him, most notably Antoine and Marie-Ann Lavoisier. This scientific couple confirmed beyond a doubt the law of conservation of mass by performing careful experiments that showed that, although matter can change its state in a chemical reaction, the total mass of matter is the same at the end as at the beginning of every chemical change. Another important contribution this couple added to the advancement of our understanding of compounds was the law of definite proportions. This law states that when a compound is formed from the chemical reaction of two elements, the elements combine in definite, fixed proportions by mass. Overview and Objectives In this lab work, you’ll react zinc and zinc oxide each with hydrochloric acid. The reaction of either zinc or zinc oxide with hydrochloric acid produces zinc chloride. You will do this work quantitatively, carefully keeping track of the masses you start with and end with. You will use the results of this investigation to experimentally calculate the mass ratios of the elements in the compounds zinc chloride and zinc oxide, and then to identify the formulas of these compounds. Practicing the calculations (You will do this while you wait for your solutions to evaporate.) The mass ratio of two elements in a compound is related to the mass ratio of the elements in that compound by the following: (atom ratio in compound) x (atomic mass ratio of elements) = mass ratio in compound To see how this is put to use, perform the following calculations: Consider the synthesis of water, which is formed in the explosive reaction of hydrogen and oxygen. (Explosions… yippee!) Let’s imagine that in a certain experiment 1.02 grams of hydrogen was reacted with excess oxygen (i.e. with enough oxygen to ensure that all the hydrogen reacts) and that 9.08 grams of water was formed. According to your data, what is the mass of hydrogen in your water sample? (This is a simple subtraction…) Use the data you now have to calculate the mass ratio of oxygen to hydrogen in water. You know that the atom ratio is 1:2 (i.e. the formula of water is H2O). You can now use the formula above to determine the atomic mass ratio of the elements. Now take a look at the periodic table. Let’s assume the mass of a hydrogen atom is one “unit.” What value in the oxygen “box” seems to represent the correct mass of oxygen? Now take a look at the same location within the hydrogen “box”… was it okay to assume the mass of a hydrogen atom is one unit? Procedure 1. Use a sharpie to label two 150 mL beakers, and then measure each beaker’s mass. Record values below. 2. Tare the beakers individually (that means, to “zero” the balance while the beaker is on the pan). a. Into the first beaker, mass out about 0.50 g of zinc by adding pieces of zinc until you get close to 0.5 grams. Then place the cover back on, wait for the reading to stabilize, and record the exact mass of your sample. b. Into the second beaker, repeat this procedure, but use zinc oxide instead of zinc. 3. Use a graduated cylinder to measure out 15 mL of 6 M hydrochloric acid solution, HCl(aq), (note that 6 M is a measure of how concentrated the HCl solution is – 6 M is fairly concentrated, so be careful and keep your safety goggles over your eyes!). 4. Add the HCl(aq) to the Zn in the first beaker. Observe the reaction and record what you see. 5. Measure the same amount of HCl and add it to the second beaker. 6. Once the reactions appear to have finished, go to the fume hood and place both beakers on the hot plate. This will drive off the excess HCl(aq), evaporate the water, and leave you with zinc chloride in each beaker 7. Determine the masses of the beakers, which now contain solid, dried zinc chloride residue. DATA TABLE Reaction #1: zinc Mass of beaker Mass of solid reactant (zinc or zinc oxide) Mass of beaker plus residue product (zinc chloride) Calculated mass of residue product (zinc chloride) Observations: Reaction #2: zinc oxide Analysis 1. Determining the correct formula for zinc chloride. a. Use the data from reaction #1 to calculate the mass of chlorine present in zinc chloride. (Note: Law of conservation of mass…this is a simple subtraction) b. Use this information to calculate the mass ratio of zinc to chlorine in the compound zinc chloride. c. If the formula for zinc chloride is ZnCl, the atom ratio of zinc to chlorine is 1:1. For this possibility, what then would the atomic mass ratio of the elements zinc to chlorine be? (You will need that equation on the first page, right?) d. Using the same logic, calculate what the atomic mass ratio of zinc to chlorine would be if the formula of zinc chloride is ZnCl2. e. Now look at a periodic table to determine the true atomic mass ratio of the elements zinc to chlorine. Compare this value to your answers to (c) and (d) to identify the correct formula. 2. Determining the correct formula for zinc oxide. You are on your own for this one, but to get you started, you will find the percent of zinc present in your zinc chloride from reaction #1 helpful. Remember – Law of constant composition. That percent should be the same in both residues. Assume three possible formulas for zinc oxide: Zn2O, ZnO, and ZnO2. 3. Explain how by experimentally determining the atomic mass ratios of Zn to Cl and Zn to O, you have indirectly determined the mass ratio of Cl to O. Use words and some algebra to show this. Thought Questions 4. What evidence do you have from your work in the lab that the Zn/HCl mixture underwent a chemical change, and not just a physical change? Be sure to explain how you distinguish between these two in your answer. 5. (This one is harder…) What evidence do you have from your work in the lab that the zinc oxide/HCl mixture underwent a chemical change, and not just a physical change? 6. Write a balanced chemical equation for each of these reactions, including states. You will have to figure out where the extra atoms go… Extension Questions (YOUR OWN WORK – no collaboration) Using the same analysis methods as you did for this lab, answer the following questions. You will need a periodic table. 1. Elements X and Y form a compound in which there is one atom of X for every four atoms of Y. When these elements react, it is found that 1.00 g of X combines with 5.07 g of Y. When 1.00 g of X combines with 1.14 g of O, it forms a compound containing two atoms of O for each atom of X. Identify X and Y. (Hint: what is the atomic mass of X… of Y?) 2. A compound of iron and oxygen contains a mass ratio of 2.616 g Fe to 1.000 g O. What is the atom ratio of oxygen to iron (and thus the formula) of this compound? ________________________________________________________________________ Hand-In Assignment Data table, well-labeled with units on every recorded measurement – TYPED. Analysis and Results – hand-written, legible. Remember to provide units and a label on every value as you present your calculations. For example: 0.514 g Zn (not just 0.514 or 0.514 g) Answers to the thought and extension questions – hand-written, legible. Collaboration: Your answers to the extension questions should be YOUR OWN WORK. You may compare your analysis and results work with your partner, but the written analysis itself should be in your own words.