Water Properties / Water Cycle / Distribution of Water / River Basins
advertisement

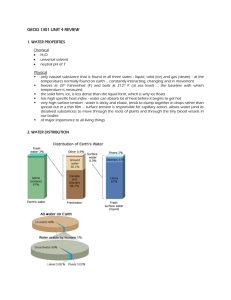
Water Properties / Water Cycle / Distribution of Water / River Basins Test Study Sheet Test Feb. 28th, 2013 The unique properties of water. o Water's molecular structure is the primary reason for all of it's unique properties. Molecules with uneven charges are called “polar” molecules. o Cohesion is the property of water that causes it to be attracted to itself. o Surface tension is a special case of cohesion. o Adhesion is when water molecules are attracted to other materials. o Capillary Action gives water the ability to “climb” structures. Water is known as the Universal Solvent o Solute + Solvent = Solution o Solute – substance dissolved in a solvent to form a solution o Solvent – fluid that dissolves solutes o Water dissolves so many substances because it is a polar molecule. o Hydrophilic – likes water (Ionic and polar molecules will dissolve) (ex. salt, Kool-Aid) o Hydrophobic – doesn't like water non-polar compounds are hydrophobic. (ex. oil) Density o Water is less dense as a solid! o Ice floats in water because it is less dense than water. o Ice sinks in rubbing alcohol because it is more dense than rubbing alcohol. o Ice forms on the surface of water first because of density. o The coldest most dense water sinks, and the less dense ice floats. Buoyancy o We feel lighter in water because of the buoyant force. o The buoyant force acts in an upward direction, opposite that of gravity. o Buoyancy is a result of density. o Objects more dense than water (like lead) will sink; o Objects less dense than water (like cork) will float; o Objects of the same density will remain at the same level (hover) and neither sink nor float. The Water Cycle o Also know as the hydrologic cycle. o A closed system meaning water is neither gained nor lost, only recycled. o There is more evaporation than precipitation over the surface of the Earth’s oceans. o There is more precipitation than evaporation over the surface of the o o o o o o o o o o o Earth’s continents. Ocean water is constantly evaporating into the atmosphere (becomes water vapor) When water vapor cools: Forms clouds at higher altitudes Forms fog if close to the ground Precipitation - water that falls to the surface from the atmosphere as rain, snow, hail, or sleet After the precipitation water can take different routes: –Evaporate –Ground Water –Soil Moisture –Surface runoff Evaporation - Water moves into the atmosphere as a gas(water vapor). The higher the temperature, the faster evaporation occurs. Ground Water - Water soaks into the ground through a process called infiltration until it reaches a zone where all of the pores are filled with water. Example: underground streams, aquifers Infiltration - important process where rain water soaks into the ground, through the soil and underlying rock layers. Some of this water ultimately returns to the surface at springs or in low spots downhill. Soil Moisture - Water that remains in the surface layer of soil, the roots of plants absorb this water. Travels up through the stems and branches of the plant into the leaves and is released into the atmosphere as vapor in a process called transpiration. Surface Runoff - Water that flows downhill into streams and rivers. Eventually empties into the ocean. Earth’s Water Supply o The total amount of water on the Earth has not changed much since early in its history. o The same water is cycled over and over. o The water you drink at lunch was probably drunk by a dinosaur millions of years ago! o 97% of Earth’s water is in the oceans o 3% of Earth’s water is fresh. o Of that 3%, most water is trapped in ice at the poles. o Most fresh water on Earth is found underground. River Basins o River Basin’s provide water to the people that live in that area. o The majority of fresh water in NC can be found in rivers