ACGS Heads of Service Meeting - Association for Clinical Genetic
advertisement
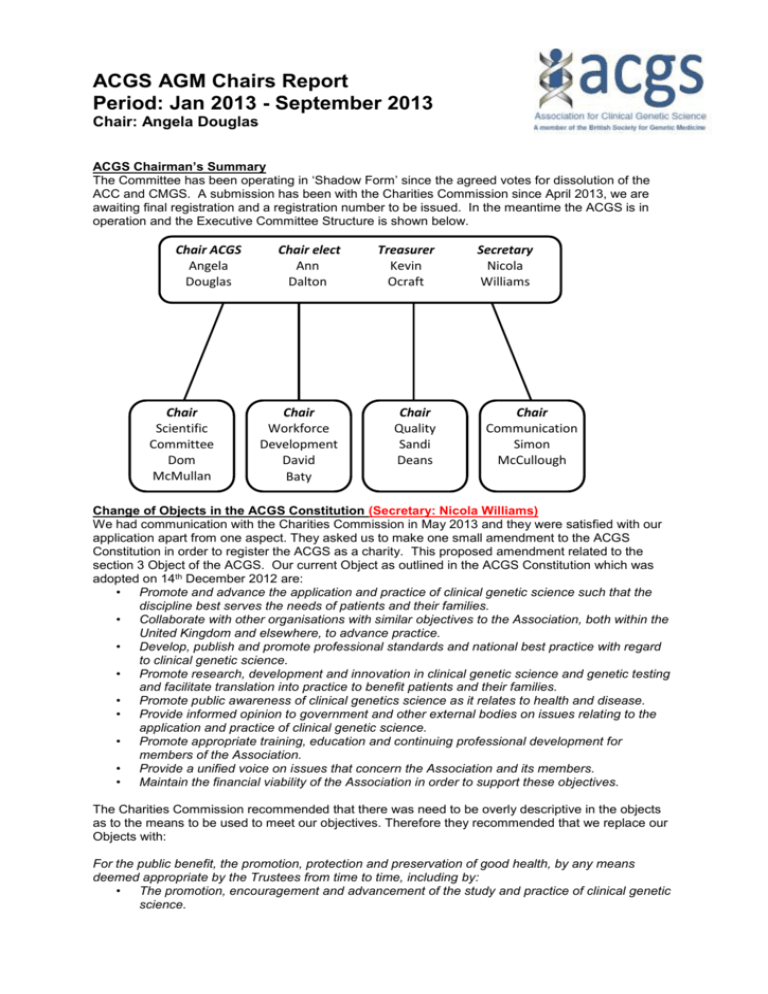
ACGS AGM Chairs Report Period: Jan 2013 - September 2013 Chair: Angela Douglas ACGS Chairman’s Summary The Committee has been operating in ‘Shadow Form’ since the agreed votes for dissolution of the ACC and CMGS. A submission has been with the Charities Commission since April 2013, we are awaiting final registration and a registration number to be issued. In the meantime the ACGS is in operation and the Executive Committee Structure is shown below. Chair ACGS Angela Douglas Chair Scientific Committee Dom McMullan Chair elect Ann Dalton Treasurer Kevin Ocraft Chair Workforce Development David Baty Chair Quality Sandi Deans Secretary Nicola Williams Chair Communication Simon McCullough Change of Objects in the ACGS Constitution (Secretary: Nicola Williams) We had communication with the Charities Commission in May 2013 and they were satisfied with our application apart from one aspect. They asked us to make one small amendment to the ACGS Constitution in order to register the ACGS as a charity. This proposed amendment related to the section 3 Object of the ACGS. Our current Object as outlined in the ACGS Constitution which was adopted on 14th December 2012 are: • Promote and advance the application and practice of clinical genetic science such that the discipline best serves the needs of patients and their families. • Collaborate with other organisations with similar objectives to the Association, both within the United Kingdom and elsewhere, to advance practice. • Develop, publish and promote professional standards and national best practice with regard to clinical genetic science. • Promote research, development and innovation in clinical genetic science and genetic testing and facilitate translation into practice to benefit patients and their families. • Promote public awareness of clinical genetics science as it relates to health and disease. • Provide informed opinion to government and other external bodies on issues relating to the application and practice of clinical genetic science. • Promote appropriate training, education and continuing professional development for members of the Association. • Provide a unified voice on issues that concern the Association and its members. • Maintain the financial viability of the Association in order to support these objectives. The Charities Commission recommended that there was need to be overly descriptive in the objects as to the means to be used to meet our objectives. Therefore they recommended that we replace our Objects with: For the public benefit, the promotion, protection and preservation of good health, by any means deemed appropriate by the Trustees from time to time, including by: • The promotion, encouragement and advancement of the study and practice of clinical genetic science. • • The advancement of education, research and innovation in clinical genetic science. The development and promotion of clinical standards in clinical genetic science. In order to make amendments to the existing ACGS Constitution, the ACGS Executive Committee proposed a resolution and the two third of the voting membership agreed to adopt this resolution. This process is outlined in section 7:1(d) of the existing ACGS Constitution (see below). Section7:1(d) ‘Any resolution to amend the provision of Part 1 of this Constitution is passed by not less than two thirds of the members present and voting at a general meeting or by post or electronic means.’ The ACGS Executive Committee proposed the resolution to change our Objects in the current constitution, to those recommended by the Charities Commission. All members of the ACGS received an email regarding this proposed resolution, and were asked to cast their vote. Voting was electronic using Survey Monkey and as with all previous electronic voting, each ACGS member was sent a unique voting number to ensure all voting was anonymous. The results of the voting were in favour of the change and the changes were submitted to the charities commission. Communications Subcommittee Report (Chair: Simon McCullough) The Communications subcommittee has 6 enthusiastic members with an interest in improving communication with the association’s members and the raising the profile of the ACGS. The committee met on the 17th June 2013 at St. Mary’s Hospital in Manchester to discuss the remit of the new committee. The initial tasks identified are summarised below: Website development This is the first priority of the communications committee and we are in the process of populating the ACGS pages. The website is operational with the address www.acgs.uk.com we would encourage you to start using the new site. All current best practice guidelines are on the new site and all new events and jobs are only going to be advertised on the new site. We are also currently deciding on which items from the ACC and CMGS websites need archived on the new site. Newsletter The communications committee now has the responsibility for the ACGS section of the newsletter. We want to have articles of interest to scientists and technologists in both molecular and cytogenetic labs. The next edition is due in January and the closing date for submission of articles will be the end of November. Please send any articles to Emma Huxley or Martin Schwarz. ACGS road show/ BSGM conference stand One of the main aims of the Communications committee is to improve communication with ACGS members and keep them up to date with developments within the profession. We also want to raise the profile of the ACGS and encourage more GTs/PTPs to become members and participate in the association. In order to do this we intend to organise a roadshow, after this conference, to visit UK genetics labs to promote the work and membership of the ACGS. We also have a stand at this conference (stand 33) where you can find out more information about the ACGS. Please do come and visit us at the stand. Simon McCullough would like to thank the committee members for their help preparing the materials for the conference and Chris Kettle for his work in uploading documents on to the new site. If anyone is interested in joining the committee particularly genetic technologists with a molecular background, please contact Simon McCullough. Quality Subcommittee Report (Chair: Sandi Deans) The newly formed Quality Subcommittee is comprised of 19 enthusiastic members from a wide range of disciplines encompassed by the ACGS, and includes individuals with various roles in ACGS laboratories from Heads of Service, Clinical Scientists, Quality Managers and EQA providers. The first meeting of the Quality Subcommittee was held at Guy’s Hospital, London on 21st June, 2013 and the remit of the group and initial tasks were identified (summarised below): • • • Audit/Activity data – Continue with data collection in CMGS format for 2012-13 period – 2013 pilot run to collect data for QF-PCR for aneuploidy from all laboratories – Collect all activity data for 2013-14 from all laboratories – This collection of data will enable more auditing for the benefit of ACGS laboratories Promote Best Practice – Currently reviewing all BPGs – Set up pipeline for BPGs to be reviewed and maintained in a timely manner – Aim for harmonisation across genetics laboratories – Provide General Genetics Reporting Guidelines workshop – 11th October, 2013 – Provide ACGS/UKGTN workshop for Next Generation Sequencing – 13th November, 2013 – Accreditation workshop for new standards as applied to genetics laboratories – Details to be confirmed Standards/Governance – Communication with appropriate bodies to help deliver and maintain a high standard of service e.g. RCPath, UKNSPC, IBMS, FASP, UKAS, UKGTN If anyone is interested in joining the Quality Subcommittee then please contact Sandi Deans on Sandi.Deans@ed.ac.uk We are particularly keen to have representation from Genetic Technologists and Trainees. Scientific Subcommittee Report (Chair: Dom McMullan) The newly formed Scientific Subcommittee is also composed of 19 enthusiastic volunteers with a highly apt range of backgrounds, reflecting cytogenetics, molecular genetics, germline and somatic interests and experiences. We are particularly keen to hear from any GTs out there interested in joining the group. The group held a first meeting on the 7th July in Birmingham and the remit and scope of this new committee was discussed and agreed. Some initial tasks under these areas were also identified for taking forward. • Conferences – To organise and theme a scientific programme for an ACGS meeting in 2014 – To determine the need and organise content of ASGS Study Days – To provide significant input into future BSGM meeting programmes • Research and Collaboration – To scope the challenges faced by labs in terms of contributing data to public databases and encourage and facilitate wider contribution – Identify the potential for ACGS Research fund awards to promote and facilitate springboard research project efforts from within Clinical Diagnostic labs – Increase contribution International initiatives (e.g. NCBI/ISCA Gene Dosage Curation process) as a collective UK/ACGS effort • Publications – Identify areas and provide the networks for pooling data into UK/ACGS publications, capitalising on our large datasets and collaborative nature If anyone has ideas for projects and meetings that the Scientific Committee should take promote and help to facilitate, please contact Dom McMullan (dominic.mcmullan@bwhct.nhs.uk). The next meeting will be held in October 2013 Workforce Development Subcommittee Report (Chair: David Baty) The new Workforce Development Subcommittee held its first meeting in Birmingham on the 18th of June 2013 and papers from this meeting are available on the ACGS web pages. The committee is comprised of eighteen members and three advisors all of whom are ACGS members with a strong commitment to training and developing the workforce. The composition of the committee is representative of the ACGS membership; Service Heads, Clinical Scientists, Senior Technologists and Training Officers. We will ensure that all groups of staff in genetics are appropriately represented on the committee as it moves forward with its work. The new committee wishes to express its thanks to the GETB and its members and is committed to continuing the work of the GETB. It is therefore extremely heartening that many GETB members are now members of the Workforce Development subcommittee. At the first meeting in June, the remit of the group was finalised and the following task identified: • • • Workforce – Gathering workforce data from all UK laboratories for 2012/2013. – Involved in drafting standards for the AHCS practitioner voluntary register – Respond to consultation on standards – Investigating route to registration for Technologists Training – Working group established for career framework 2-4 staff in genetics – Seek feedback from labs delivering new STP Clinical Bioinformatics – Greater involvement in OSFA process – Organise training meetings – Investigate lead TO role Stakeholders – Collaborate with key stakeholders e.g. ACGS members, NSHCS, AHCS, RCPath, IBMS, VRC and the MSC team. The next meeting of the committee will take place in November and we are particularly keen to identify areas of work that members wish progressed. Please email your comments and suggestions to David Baty (dbaty@nhs.net) GETB letter from Jennie Bell Report from GETB to ACGS Executive Committee June 2013 The Genetics Education and Training Board (GETB) held its first meeting in Birmingham on 5 th August 2010. This meeting was the joining of the two committees that represented the Molecular and Cytogenetic training needs of the workforce. It was the first committee to function as a fully merged professional group. Since 2010 the GETB, as it was named, offered support to the training schemes for Scientists in Molecular and Cytogenetics, the national training programme for Genetic Technologists, the developing Modernising Scientific Careers in Genetics training programmes (STP and PTP) and the consultation on career frameworks 2 to 4. In addition the committee collated workforce intelligence data, supported FRCPath preparation (Part and 2), engaged in discussions regarding registration and regulation of all staff and generally represented the training needs of the Profession in every reasonable capacity at the national level. The success of this committee, as demonstrated by high calibre trainees exiting all of the supported training programmes and a skilled and committed Genetics workforce, was predominantly the result of the commitment and hard work of the committee members. In particular, the leadership shown by the first chair and deputy chair (David Bourn and Gordon Lowther) made sure that the committee fulfilled the expanding and demanding remit. The committee was composed of senior members of the Scientific and Technical workforce from both areas of the discipline, all of whom have a wealth of knowledge and experience in training delivery. It is with some sadness that we now request that the ACGS Executive committee accept the disbanding of the GETB. However, the entire GETB we would like to offer their full support to the new Workforce and Development Committee (meeting for the first time on 18th June 2013). The final meeting of GETB took place on 15th March 2013. The agenda for our workforce development continues to expand, particularly with regard to registration, the career framework 2 to 4 strategy, Higher Specialist training and the development of the Bioinformatics STP. The new committee will need to expand to meet these demands and to also support the creation of the next generation of leaders in workforce development. On behalf of our entire workforce I would like to thank all of those training committee members (ACC/ETC, CMGS/TAB and GETB) who have over the many years given their time and energy to support the development of our staff in what is a continually changing and challenging environment. Without this commitment our profession would be severely lacking. Jennie Bell June 2013 On behalf of the Committees of the ACGS, ACC and CMGS, I would like to extend our sincere gratitude towards all the present and past members of the GETB for all the hard work and dedication they have put into making the GETB the success that it was, without which, the Profession of Genetic Scientists would not have the excellent training programmes and educational support it has the privilege of enjoying today. I would especially extend our thanks to Gordon Lowther, David Bourn and Jennie Bell; for all that they have contributed to the GETB for the Professional Associations over the past years and echo Jennie’s words above “Without this commitment our profession would be severely lacking”. Thank you all. Head of Service Meeting This was held in April 2013 in Manchester notes and presentations from this meeting can be found on the website. This is an important chance to meet, network and discuss topical issues and the ACGS will be hosting another HOS meeting again next year, perhaps over 2 days to allow for greater discussion/networking. We will keep you informed and issue a date well in advance to ensure good attendance. 100K Genome Project and Laboratory Reconfiguration The report in January 2012 by the Human Genomics Strategy Group (HGSG), ‘Building on our inheritance - genomic technology in healthcare’ challenged the NHS to consider significant modernisation and development of genetic testing services through a network structure. In January 2013 the 100k genome project was launched with the remit of delivering 100K genomes for £100M. The responsibility for the delivery of this was given to the NHS England Genomics Strategy Board, chaired by Sir Malcolm Grant. More recently the Board have also been tasked with overseeing the rationalisation and reconfiguration of existing genetic testing services, in line with the HGSG proposed network of regional genetic centres, biomedical diagnostic hubs and genomic technology centres. It is clear that it is intended that the overall number of genetic testing laboratories will be decreased with each remaining facility providing a broader range of genetic analysis. NHS England is the direct commissioner for medical genetics services and has published the first national specification http://www.england.nhs.uk/npc-crg/group-e/e01/. From NHS England: The Genomics Strategy Board, hosted by NHS England and chaired by Sir Malcolm Grant, was tasked with providing guidance on the delivery of the Prime Minister’s ambition on genomics to deliver the procurement of 100k genomes. The Genomics Strategy Board has met six times and concluded its work on 28 June 2013, with recommendations transferred to the newly established Genomics England Limited. Prior to the establishment of the 100k genome project, the DH established the Human Genome Strategy Group (HGSG). Over a number of months the HGSG made a series of recommendations, some of which were referred to NHS England to consider. It is proposed that NHS England specialised services leads the re-design of genetic testing services through the medical genetics Clinical Reference Group. The model proposed in the HGSG, and subject to approval by the NHS England Board, has three components: Genomic technology centres could operate as specialist centres of excellence with a focus on the interface between translational research and service innovation in genomic services. They could bring together clinical, academic, scientific and bioinformatics specialists to translate cutting-edge research in a collaborative and inclusive manner to ensure the participation of specialist expertise and promote the adoption and spread of research and innovation. They could play a key role in evaluating new markers for cost and clinical effectiveness. A key requirement is that these organisations should be designated as genomic technology centres through open competition against a specification, and commissioning would be through NHS England. Biomedical diagnostic hubs with a strong integrated molecular capability could be developed to incorporate all current laboratory-based diagnostic services in pathology and genetics (inherited and acquired diseases). They are likely to be regional/network hubs of significant scale, and are emerging from the national pathology transformation programme. These hubs could operate as the essential interface between the clinician and the pathologist for rapid and appropriate testing, particularly where co-ordination of sample processing and analysis is crucial. The exact number of such laboratories and the scope of testing to be undertaken requires further development but is likely to include high throughput analysis, frequently requested biomarkers and, for example, molecular tests for microbiology, virology and haematology. Regional genetics centres will continue to have an important role in the diagnosis of inherited disorders and the management of familial aspects of disease. They will continue to provide a key interface with patients with genetic disease. Clinical genetics services could have an expanded role, in partnership with specialist clinicians, to provide genetic expertise as genetic services are expanded and embedded in clinical pathways. As clinicians in other specialties become more proficient and the number and range of specialties involved continues to expand, it is envisaged that the relationship between regional genetics centres and other specialties will evolve to one which provides leadership, expert support and mentoring, and management of particular family issues such as reproductive counselling. Progress to date The DH has established the company ‘Genomics England’. The Secretary of State announced on the 5 July that Sir John Chisholm has been appointed Chair of the company Board. The remainder of the Board will now be appointed with a range of business and clinical expertise, including representative from NHS England. The Board will be responsible for the delivery of the business plan; including managing procurement contracts and taking operational decisions associated with the general running of the business. Genomics England Ltd is intended as a vehicle for delivery and the specific structure of the company and function is yet to be determined. Further work to underpin the business model of Genomics England Ltd. is currently taking place. NHS England does recognise that the status quo of existing genetic testing services will be inadequate for the future. NHS England commissioned PA Consulting, on behalf of the Genomics Strategy Board, to embark on a benefits realisation proposition which could underpin a business plan and operating model of the procurement vehicle. With the establishment of Genomics England Limited, this work will be transferred to that forum. In light of the recommendations by the HGSG, rationalisation and reconfiguration of existing genetic testing services will enable optimal benefits realisation. To deliver the service model change a project brief has been developed by the specialised services national programme of care using the framework established for all direct commissioning specialised services. A revised service specification is planned to be developed for the genomic technology centres, the biomedical diagnostic hubs and the regional genetics centres. A growing suite of national commissioning policies will be developed. The proposed project brief identifies a process that procures the revised service structures for a launch date in January 2015. NHS England is responsible for service commissioning elements and will establish a formal service reconfiguration steering group as part of the medical genetics CRG. It will structure to oversee the reconfiguration project using the governance of the Clinical Priorities Advisory Group and direct commissioning committee to secure timely delivery. UKGTN CSAG The remit of the UKGTN Clinical and Scientific Advisory Group is to establish principles, define assessment criteria and influence mechanisms to inform the commissioning and provision of quality genetic laboratory services in the UK for NHS patients. The UKGTN CSAG meets twice a year, usually in March and September. The September meeting includes the recommendations of tests that have been evaluated by the Genetic Test Evaluation Working Group. Tests that are approved will be included in the NHS Directory of Genetic Disorders/Genes for Diagnostic Testing and added to the on-line database of laboratory services. The recommended tests will be submitted to NHS England through the Medical Genetics Clinical Reference Group to be considered for NHS resources. The March 2013 meeting reviewed the previous year’s annual work programme and agreed the following years work programme. Members of UKGTN CSAG and its working groups have a collective responsibility for participating in discussions on the work of the UKGTN. We have two members from the ACGS represented on UKGTN CSAG. Each member has a duty to provide expert input on topics related to their discipline to the best of their knowledge and ability, and to make the CSAG aware of the full range of opinions within their discipline. Members are also expected to contribute towards the collective determination of the CSAG's view on matters outside their specific area of expertise. Areas of discussion more recently with UKGTN have involved the Genetic Dashboard, and changing the format of the Gene dossiers in line with new technologies, a meeting to be held in October will provide more detail. RCPath SAC for Genetics & Clinical Embryology Both Katie Waters and Gail Norbury are due to demit their roles as ACC and CMGS representatives on the committee. In her capacity as Chair, Gail is thus seeking nominations from the ACGS for two new representatives. These are 3-year terms. Gail and I are keen to strengthen the links with molecular pathology and I would like to suggest that Rachel Butler as a good candidate for one of these posts as she also sits on the molecular pathology inter-specialty committee. It would then make sense for the other representative to have a stronger cytogenetic background. Rachel has agreed we are still looking for a representative with a Cytogenetic background to take on the vacant position. There are two formal meetings a year. The next meeting is not until 9 December 2013 at 1.00 p.m. However, Council is delegating more work to the SAC so nominees need to commit to helping prepare and update various documents, arranging symposia and attending the meetings. NQAAP Prof Mike Griffiths has written as chair of the National Quality Assessment Advisory Panel (NQAAP) for Genetics with respect to this committee and notice of standing down. The panel has recently consisted of 2 ACC representatives (Paul Roberts and Kim Smith), 2 CMGS representatives (Dave Robinson and Fiona Macdonald), and 1 each for AGTC, ACB, IBMS and BSH (together with scheme organisers in an advisory capacity). The replacement of the ACC and CMGS by the ACGS is an opportunity to review the representation of genetic laboratories on this panel; however, Mike suggests at this stage we simply continue with four ACGS nominees to maintain some continuity. For 2014 we will need to replace: Dave Robinson - who is retiring and does not wish to continue beyond his current 3 year term at the end of 2013. Kim Smith - who has continued beyond retirement but now feels it is time to stand down from the end of 2013 (after one year of her second 3 year term). Mike has also indicated and confirmed his wish to stand down as Chair of NQAAP at the end of his current three year term (this year). The panel currently meets twice a year (March/April & October/November). The principle work of the panel is dealing with issues of persistent poor performance identified though NEQAS. The panel also has a general oversight role in all EQA in Genetics. The chair of the panel sits on the Joint Working Group for Quality Assurance along with NQAAP chairs from all other areas of Pathology. Please could members give thought to becoming one of two ACGS representatives to fulfil the NQAAP roles. Nominees should ideally have a background in EQA and sufficient seniority and experience to be able to support the chair in dealing with senior staff in a poorly performing laboratory. One of these would need to be willing to fulfil the recent requirement to identify a Chair Elect (there are no volunteers from within current membership of NQAAP). We need to propose the new Chair to RCPath. Ros Hastings and Sandi Deans may be able to offer advice on suitable candidates Pathology Quality Assurance Review As you may be aware, late last year Prof Sir Bruce Keogh asked Dr Ian Barnes to chair a review into quality assurance practice and governance within pathology (https://www.gov.uk/government/news/pathology-quality-assurance-review-launched), which is now well underway. One of the facets of the Review was to look at the extent to which QA training is built into job plans and the emphasis it is given in the working life of pathology scientists and medics at different stages in their careers. In order to collect data on this a short questionnaire has been developed, accessible via the link below. http://www.ibms.org/go/media/news,401 As graduates of the Influencing the Future programme, and key players in the shaping of the future of services, Ian was very keen to see our views and experience included. We were asked to complete the questionnaire and disseminate it as widely as possible amongst colleagues in our organisation, which we did. A representative group of the ACGS also met with Dr Barnes and following discussions, submitted a response on behalf of Genetic Scientists informing of the internal and external quality measures undertaken by Genetics laboratories to assure safe practices, the group consisted of Sandi Deans, Chair of ACGS Quality Committee, Mike Griffiths, Chair NQAAP, Rob Elles, EQN and Angela Douglas, Chair ACGS. As part of the Pathology Quality Assurance Review Dr Barnes is planning a series of workshop events, one of which we have been asked to attend. The purpose of this event is to review the early findings emerging from the Pathology Quality Assurance Review and consider their implications for future work by the professional associations and their membership. Delegates should be able to speak for their association and should be actively engaged in clinical practice, therefore both Sandi and I will be attending the meeting will take place on Thursday 31st October 2013 at 10am-4pm, in London (venue TBC). Prepared by: Angela Douglas Chairman September 2013