Review Lecture Worksheet (the answers are written in white font
advertisement

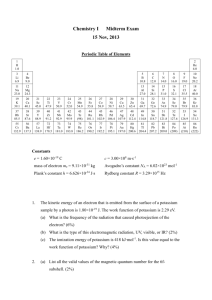
Review Lecture Worksheet (the answers are written in white font, switch all font to black to view them) Rank the 3rd period p- block elements (In order of atomic number that is: Na, Mg, Al, Si, P, S, Cl, ArAr) in terms of electron affinity, electronegativity and ionization energy. Explain all. Explanation: of general trend. As you go across the periodic table toward the noble gasses, effective nuclear charge increases. This causes all three of the trends listed above to increase as well. Ionization Energy: Low-High: Na, Al, Mg, Si, S, P, Cl Explanation of exceptions: because Mg and P have stable filled and half-filled subshells respectively they have an abnormally high ionization energy. Causing them to “switch” orders with the Al and S respectively. Electron affinity: Low-High: Mg, Na, Al, P, Si, S, Cl Explanation of exceptions: because Mg and P have stable filled and half-filled subshells respectively they have an abnormally low electron affinities. Causing them to “switch” orders with the Na and Si respectively. Electronegativity: Low-High: Na, Mg, Al, Si, P, S, Cl Explanation of exceptions: because electronegativity is something we discuss with atoms in a bond, all the electron configurations are already stable, leaving electronegativity to only be related to the effective nuclear charge and not to the electron configurations. Draw ICl4- and ICl4+. For each- What is electron geometry, molecular geometry, bond angles, hybridization of the central atoms. What orbital does each atom use to bond with? (answer on next page) Draw the MO diagram for CO assume that 2p is higher in energy than 2p. Be sure to label all atomic and molecular orbitals, including subscripts. Be sure to fill in all electrons necessary. What is the bond order? Is it paramagnetic or diamagnetic? (answer on next page) How does constructive and destructive interference relate to molecular orbital diagrams? B.O.: ½ (8-2)=3 diamagnetic Constructive interference forms bonding orbitals as shown by the overlap in the picture to the left. Destructive interference forms antibonding orbitals as shown by the overlap in the picture to the left. What is the molecular formula for the following? Give the hybridization of circled atoms (for the sake of practice do it for all of them till you get the hang of it, any are fair game). How many sigma bonds are present? How many pi bonds are present? Any guess what molecule it is? C17H13ClN4 (hints: be careful to get the carbon at the end of the line, up near the top. Don’t forget it also has 3 hydrogens attached). Hybridization: most common mistake here is on the nitrogens, don’t forget there is a lone pair that isn’t drawn in line structures, making the =N- nitrogens sp2 hybridized and the nitrogen with three single bonds sp3 hybridized. Sigma bonds: 25 pi bonds present: 9 The molecule is Alprazolam, better known as xanex (I thought it appropriate for a review sheet) Rank the following molecules in order of bond angles: H2O, CH4, NH3, BH3, NO2, NO2-. For each molecule state the hybridization and what orbital each bond is formed with, as well as where the lone pairs on the central atom is (are) located. For H2O, ClO3- and NO2 rank in order of expected bond length and strength. H2O, ClO3-, CH4, NO2-, NO2, BH3 Bond orders: H2O= 1 NO2=1.5 ClO3-= 5/3=1.666 As bond order increases length decreases and strength increases. Length: ClO3-< NO2< H2O Strength: ClO3->NO2>H2O