Supplemental File S11. miRNAs in Humans
advertisement
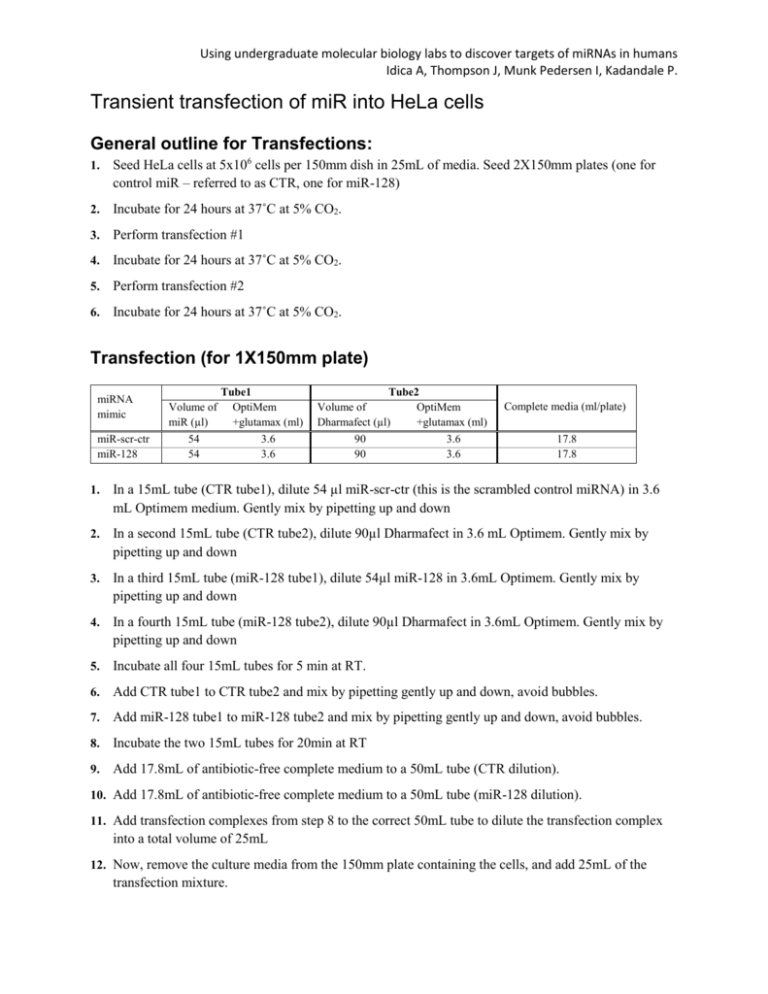
Using undergraduate molecular biology labs to discover targets of miRNAs in humans Idica A, Thompson J, Munk Pedersen I, Kadandale P. Transient transfection of miR into HeLa cells General outline for Transfections: 1. Seed HeLa cells at 5x106 cells per 150mm dish in 25mL of media. Seed 2X150mm plates (one for control miR – referred to as CTR, one for miR-128) 2. Incubate for 24 hours at 37˚C at 5% CO2. 3. Perform transfection #1 4. Incubate for 24 hours at 37˚C at 5% CO2. 5. Perform transfection #2 6. Incubate for 24 hours at 37˚C at 5% CO2. Transfection (for 1X150mm plate) miRNA mimic miR-scr-ctr miR-128 Volume of miR (µl) 54 54 Tube1 OptiMem +glutamax (ml) 3.6 3.6 Tube2 Volume of OptiMem Dharmafect (µl) +glutamax (ml) 90 3.6 90 3.6 Complete media (ml/plate) 17.8 17.8 1. In a 15mL tube (CTR tube1), dilute 54 µl miR-scr-ctr (this is the scrambled control miRNA) in 3.6 mL Optimem medium. Gently mix by pipetting up and down 2. In a second 15mL tube (CTR tube2), dilute 90µl Dharmafect in 3.6 mL Optimem. Gently mix by pipetting up and down 3. In a third 15mL tube (miR-128 tube1), dilute 54µl miR-128 in 3.6mL Optimem. Gently mix by pipetting up and down 4. In a fourth 15mL tube (miR-128 tube2), dilute 90µl Dharmafect in 3.6mL Optimem. Gently mix by pipetting up and down 5. Incubate all four 15mL tubes for 5 min at RT. 6. Add CTR tube1 to CTR tube2 and mix by pipetting gently up and down, avoid bubbles. 7. Add miR-128 tube1 to miR-128 tube2 and mix by pipetting gently up and down, avoid bubbles. 8. Incubate the two 15mL tubes for 20min at RT 9. Add 17.8mL of antibiotic-free complete medium to a 50mL tube (CTR dilution). 10. Add 17.8mL of antibiotic-free complete medium to a 50mL tube (miR-128 dilution). 11. Add transfection complexes from step 8 to the correct 50mL tube to dilute the transfection complex into a total volume of 25mL 12. Now, remove the culture media from the 150mm plate containing the cells, and add 25mL of the transfection mixture. Using undergraduate molecular biology labs to discover targets of miRNAs in humans Idica A, Thompson J, Munk Pedersen I, Kadandale P. 13. Gently rock plate back/forward, left/right to distribute transfection mixture evenly. 14. Incubate for 24 hours at 37˚C at 5% CO2. 15. Repeat transfection procedure on these cells (transfection #2). 16. Incubate for 24 hours at 37˚C at 5% CO2. 17. Harvest and aliquot cells. Harvest and aliquot cells (the following is per treatment) 1. Preheat 0.25% trypsin (alternatively, mix cold 0.25% trypsin with room temp PBS 1:1) 2. Aspirate media from 150mm plate. 3. Rinse cells with 25mL 1X PBS (Mg and Ca free!) 4. Aspirate PBS 5. Add 5mL trypsin solution and incubate at RT for 5-7min. 6. Gently tap plate to detach any remaining cells and add 5mL complete EMEM 7. Collect cell suspension (10mL) into a 50 mL tube 8. Add 20mL complete EMEM for a total of 30mL total volume. 9. Aliquot the cell suspension into 30 tubes (1mL per tube). 10. Centrifuge for 5 minutes at 1200RPM at RT (11cm diameter rotor, swinging bucket) 11. Aspirate supernatant. 12. Snap freeze in liquid nitrogen 13. Store at -80˚C











