GM-notification_form_HY
advertisement
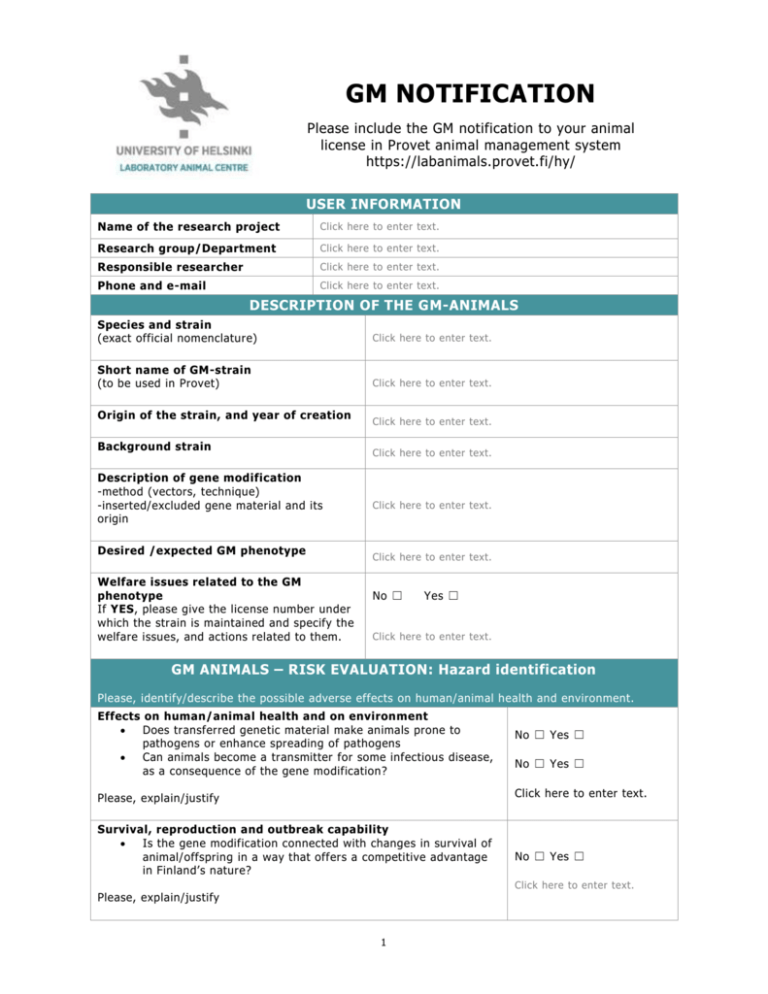
GM NOTIFICATION Please include the GM notification to your animal license in Provet animal management system https://labanimals.provet.fi/hy/ USER INFORMATION Name of the research project Click here to enter text. Research group/Department Click here to enter text. Responsible researcher Click here to enter text. Phone and e-mail Click here to enter text. DESCRIPTION OF THE GM-ANIMALS Species and strain (exact official nomenclature) Click here to enter text. Short name of GM-strain (to be used in Provet) Click here to enter text. Origin of the strain, and year of creation Background strain Description of gene modification -method (vectors, technique) -inserted/excluded gene material and its origin Desired /expected GM phenotype Welfare issues related to the GM phenotype If YES, please give the license number under which the strain is maintained and specify the welfare issues, and actions related to them. Click here to enter text. Click here to enter text. Click here to enter text. Click here to enter text. No ☐ Yes ☐ Click here to enter text. GM ANIMALS – RISK EVALUATION: Hazard identification Please, identify/describe the possible adverse effects on human/animal health and environment. Effects on human/animal health and on environment Does transferred genetic material make animals prone to pathogens or enhance spreading of pathogens Can animals become a transmitter for some infectious disease, as a consequence of the gene modification? No ☐ Yes ☐ No ☐ Yes ☐ Click here to enter text. Please, explain/justify Survival, reproduction and outbreak capability Is the gene modification connected with changes in survival of animal/offspring in a way that offers a competitive advantage in Finland’s nature? No ☐ Yes ☐ Click here to enter text. Please, explain/justify 1 GM NOTIFICATION Please include the GM notification to your animal license in Provet animal management system https://labanimals.provet.fi/hy/ Special requirements for animal care If special requirements, describe Isolation of animals Protection of personnel Waste disposal (handling of dirty bedding, fee, carcasses, etc.) Other special requirements, e.g. feeding Normal care ☐ Click here to enter text. GM ANIMALS – RISK EVALUATION: Risk evaluation (Contained use) Please, consider the hazard identified above. Estimate the risks related to them (taking into account the severity and probability). Consider how the planned preventive measures affect the risk and whether they are adequate. Based on these considerations, select the class of use. Use classification Class 1: There is no or minor risk related to the use of animals. GM animals do not cause harm to humans/environment. They are unlikely to be reproductive in Finland’s nature or to settle in there Class 1 ☐ Class 2 ☐ Class 2: There are bigger than minor risk or remarkable ethical questions related to use of animals. GM animals may cause ha rm to humans/environment. GM animals can breed in Finland’s nature. NB! Current LAC’s notification to the Board of Gene Technology covers the class 1 contained use. The use of common lab animals (as rats, mice, hamsters) can usually be included into this cl ass. A new notification/application is needed for class 2 use. RECEIPT Place and date Person who have mad the notification Click here to enter text. Click here to enter text. After the use of animals has started, the user is responsible to report to LAC, if - there is any new data/observations that might affect the risk evaluation there are deviations and/or dangerous situations that have (or might have) led to unintended spread of the GM animals inside the facility, or to their escape outside facility. The user must always describe the measures that were taken 2