Synthesis of Lead Chromate Lab
advertisement

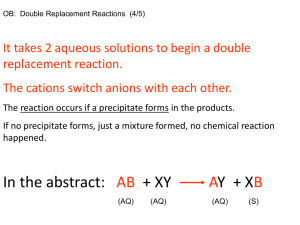
Synthesis of Lead Chromate Purpose Calculate the percent yield of lead (II) chromate obtained from the reaction of a solution of potassium chromate and a solution of lead (II) nitrate. Background Information When two chemical systems interact, sometimes the result is the formation of a precipitate. Thus, the reaction that occurs goes essentially to completion. The stoichiometry of such a reaction can be considered if solutions of known concentration are allowed to react. For example, if solutions of potassium chromate and of lead (II) nitrate are allowed to react, we can calculate the percent yield of product by finding the weight of dry lead (II) chromate obtained. The reaction involved in the experiment is shown below K2CrO4(aq) + Pb(NO3)2(aq) __________> PbCrO4(s) + 2 KNO3(aq) Inspection of the reaction reveals that one mole of potassium chromate reacts with one mole of lead (II) nitrate to form one mole of lead (II) chromate. In all experiments, the number of moles of a product formed will depend on the number of moles of the limiting reactant. The stoichiometry of this reaction is such that potassium chromate and lead (II) nitrate must react in the ratio of one mole of potassium chromate to one mole of lead (II) nitrate. If the quantity of either reactant is such that the ratio is not 1:1, the excess quantity of reactant will not react. For example, if 2 mol of potassium chromate were reacted with 1 mol of lead (II) nitrate, the potassium chromate would be present in excess, so that the number of moles of lead (II) chromate formed would depend on the number moles of lead (II) nitrate present. In this case, 1 mol of lead (II) chromate would be formed. Thus, lead (II) nitrate would be the limiting reactant for this experiment. Equations for Calculations Number of moles of reactant=(molarity of solution) x (volume of solution in liters) The percent yield of product can be found using the following equation Percent yield =(Number of grams of product formed) (Theoretical number of grams of product) x 100 Procedure Measure 50 mL of 0.10 M potassium chromate solution in a clean 100 mL graduated cylinder. Transfer this solution to a clean 250 mL beaker. Thoroughly wash the graduated cylinder with tap water.. Rinse the graduated cylinder with a 20 mL portion of distilled water so that the water contacts the entire inner surface of the cylinder. Discard the rinse water. Repeat this procedure with two additional 20 mL portions of distilled water. Discard the rinse water. Measure 50 mL of 0.11 M lead (ii) nitrate solution in a clean 100 mL graduated cylinder. With continuous stirring, using a glass rod, slowly pour the lead (II) nitrate solution into the potassium chromate solution. Continue to stir the mixture for 5 minutes. Weigh a clean, dry watch glass and a piece of filter paper. Record the total mass of the watch glass and paper on the Data Sheet. Place a clean filtering funnel in a funnel stand, or in a utility clamp attached to a ring stand. Fold a piece of filter paper as shown in figure 1. Fold the circle of filter paper in half. Make a second fold as shown so that the edges of the filter paper do not quite match, as shown, and so that the angle formed by the two edges of the paper should be about 5 to 10 degrees. Tear off a corner of the smallest section of the filter paper. Place the torn corner of the filter paper in the paper cone, since the mass of this piece of filter paper was included when the watch glass and filter paper were weighed. Place a 150 mL beaker under the funnel so that the stem of the funnel touches the side of the beaker. Moisten the filter paper in the funnel with distilled water from a wash bottle. Firmly press the edges of the filter paper against the funnel. Add sufficient distilled water to the funnel to fill the stem of the funnel. Using a medicine dropper, add a few drops of lead (II) nitrate solution to the reaction mixture. If some precipitate forms as the additional precipitant (the lead (II) nitrate solution) is added, slowly add an additional 10 mL portion of lead (II) nitrate solution to the reactant mixture while stirring the reaction mixture constantly with a glass stirring rod. Continue to stir the reaction mixture continuously for 5 minutes. Allow the precipitate to settle to the bottom of the beaker. Retest the reaction mixture with a few drops of the lead (II) nitrate solution to determine if the precipitation is complete. Decant as much of the supernatant (the liquid portion of the reaction mixture) as possible from the precipitate into the funnel. Guide the supernatant with a glass stirring rod from the beaker into the funnel as shown in figure 2. After most of the of the supernatant has been filtered, add about 20 mL of distilled water to the precipitate in the beaker by directing a stream of distilled water from a wash bottle onto the remaining precipitate in the beaker. Allow the precipitate to settle and decant the wash solution through the funnel, using the procedure previously described. Wash the precipitate with two additional portions of distilled water and each time transfer the wash solution to the funnel. After the precipitate is washed thoroughly, transfer it from the beaker to the funnel with the aid of a stream of distilled water from the wash bottle, as shown in figure 3. After washing as much of the precipitate as possible out of the beaker and into the funnel, transfer the remaining portions of the precipitate from the beaker to the funnel by using a stirring rod fitted with a rubber policeman. After the transfer of the precipitate is completed, direct a stream of distilled water from the wash bottle onto the rubber policeman, holding the attached stirring rod so that the washings go into the funnel. Using a gentle stream of distilled water from the wash bottle, wash the precipitate down the sides of the filter paper cone into the bottom of the cone. Collect 1 mL of filtrate in a test tube. To the filtrate in the test tube, add 1 mL of 0.10 M potassium chromate solution to test for lead (II) ion in the filtrate. If a yellow precipitate of lead (II) chromate forms, wash the precipitate on the filter paper by carefully adding distilled water from the wash bottle to the funnel. Retest a second sample of filtrate for lead (II) ion. If lead (II) ion is still present in the filtrate, wash the precipitate again. Allow the funnel assembly to stand until all of the liquid has passed through the filter paper. Carefully remove the filter paper and precipitate from the funnel and place the paper and precipitate on the preweighed watch glass. Carefully open the filter paper out flat on the watch glass. Label the watch glass and contents with an identifying mark. Place the watch glass and contents in an oven at 80oC for 30 minutes. Using crucible tongs, remove the watch glass and contents from the oven and allow them to cool in the air. When cool, weigh the watch glass, filter paper, and precipitate. Enter this mass on the Data Sheet. Repeat this procedure until the mass of precipitate remains constant. Calculations 1. Calculate the number of moles of potassium chromate and lead (II) nitrate used in this experiment. 2. Calculate the number of moles of lead (II) chromate formed in this experiment. 3. On the basis of the limiting reactant, calculate the theoretical number of moles of lead chromate that should have been formed in the experiment. 4. Calculate the theoretical mass of lead (II) chromate that should have been formed in the experiment. 5. Calculate the percent yield of lead (II) chromate obtained in this experiment.