Liu B - Effect of working fluids on organic Rankine cycle for waste
advertisement

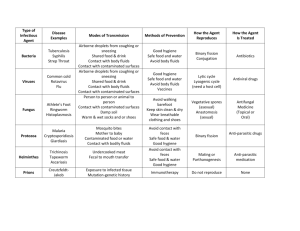
Liu BT. Effect of working fluids in organic Rankine cycle for waste heat recovery, Energy 29 (2004): 1207–1217 Abstract Analysis of the performance of an ORC influence of working fluids. Investigated effects on thermal efficiency 𝜂𝑡ℎ and total heat-recovery efficiency 𝜂𝑡 . Presence of Hydrogen bond (in H2O, NH3 and ethanol) wet fluid conditions due to >> vaporizing enthalpy inappropriate for ORC systems. Calculations: o 𝜂𝑡ℎ is a weak function of 𝑇𝑐𝑟𝑖𝑡 . o Max of 𝜂𝑡 occurs at the appropriate 𝑇𝑒𝑣𝑎𝑝 between 𝑇𝑖𝑛,𝑤𝑎𝑠𝑡𝑒 ℎ𝑒𝑎𝑡 and 𝑇𝑐𝑜𝑛𝑑 . o Max of 𝜂𝑡 ↑ if 𝑇𝑖𝑛𝑙𝑒𝑡,𝑊𝐻 ↑ o Max of 𝜂𝑡 ↓ by using working fluids with a lower 𝑇𝑐𝑟𝑖𝑡 . Results 𝜂𝑡ℎ is a weak function of 𝑇𝑐𝑟𝑖𝑡 , although 𝜂𝑡ℎ for working fluids with a lower 𝑇𝑐𝑟𝑖𝑡 is lower. Conclusion The presence of hydrogen bond in certain molecules, such as water, ammonia, and ethanol results in wet fluids due to larger vaporizing enthalpy, and is regarded as inappropriate for ORC systems. Thermal efficiency for various working fluids is a weak function of the critical temperature, regardless of the fact that the thermal efficiency for working fluids with the lower critical temperature is lower. In general, the maximum value of total heat-recovery efficiency occurs at the appropriate evaporating temperature that is between the inlet temperature of waste heat and the condensing temperature. The maximum value of total heat-recovery efficiency increases with the increase of the inlet temperature of the waste heat 𝑇𝑖𝑛𝑙𝑒𝑡,𝑤𝑎𝑠𝑡𝑒 ℎ𝑒𝑎𝑡 and decreases it by using working fluids of the lower critical temperature 𝑇𝑐𝑟𝑖𝑡 . Analysis using a constant waste heat temperature or based on thermal efficiency may result in considerable deviation for system design relative to the varying temperature conditions of the actual waste heat recovery.