BCBS GA In-House Lab List: CPT Codes & Descriptions
advertisement
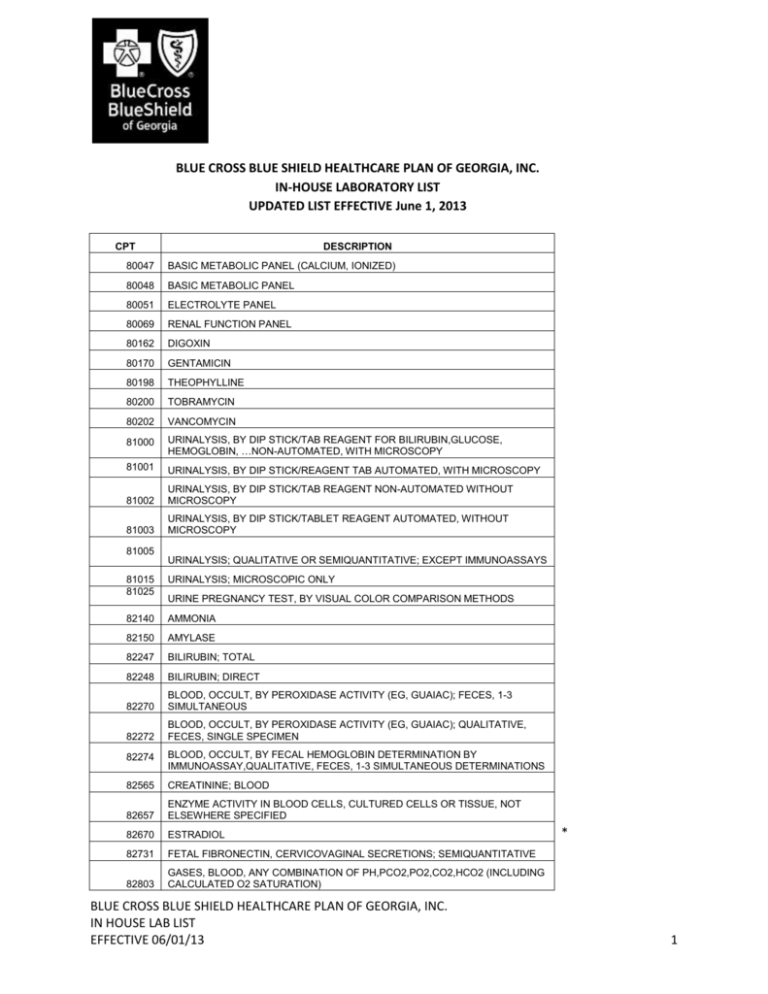
BLUE CROSS BLUE SHIELD HEALTHCARE PLAN OF GEORGIA, INC. IN-HOUSE LABORATORY LIST UPDATED LIST EFFECTIVE June 1, 2013 CPT DESCRIPTION 80047 BASIC METABOLIC PANEL (CALCIUM, IONIZED) 80048 BASIC METABOLIC PANEL 80051 ELECTROLYTE PANEL 80069 RENAL FUNCTION PANEL 80162 DIGOXIN 80170 GENTAMICIN 80198 THEOPHYLLINE 80200 TOBRAMYCIN 80202 VANCOMYCIN 81000 URINALYSIS, BY DIP STICK/TAB REAGENT FOR BILIRUBIN,GLUCOSE, HEMOGLOBIN, …NON-AUTOMATED, WITH MICROSCOPY 81001 URINALYSIS, BY DIP STICK/REAGENT TAB AUTOMATED, WITH MICROSCOPY 81002 URINALYSIS, BY DIP STICK/TAB REAGENT NON-AUTOMATED WITHOUT MICROSCOPY 81003 URINALYSIS, BY DIP STICK/TABLET REAGENT AUTOMATED, WITHOUT MICROSCOPY 81005 URINALYSIS; QUALITATIVE OR SEMIQUANTITATIVE; EXCEPT IMMUNOASSAYS 81015 81025 URINALYSIS; MICROSCOPIC ONLY 82140 AMMONIA 82150 AMYLASE 82247 BILIRUBIN; TOTAL 82248 BILIRUBIN; DIRECT 82270 BLOOD, OCCULT, BY PEROXIDASE ACTIVITY (EG, GUAIAC); FECES, 1-3 SIMULTANEOUS 82272 BLOOD, OCCULT, BY PEROXIDASE ACTIVITY (EG, GUAIAC); QUALITATIVE, FECES, SINGLE SPECIMEN URINE PREGNANCY TEST, BY VISUAL COLOR COMPARISON METHODS 82274 BLOOD, OCCULT, BY FECAL HEMOGLOBIN DETERMINATION BY IMMUNOASSAY,QUALITATIVE, FECES, 1-3 SIMULTANEOUS DETERMINATIONS 82565 CREATININE; BLOOD 82657 ENZYME ACTIVITY IN BLOOD CELLS, CULTURED CELLS OR TISSUE, NOT ELSEWHERE SPECIFIED 82670 ESTRADIOL 82731 FETAL FIBRONECTIN, CERVICOVAGINAL SECRETIONS; SEMIQUANTITATIVE 82803 GASES, BLOOD, ANY COMBINATION OF PH,PCO2,PO2,CO2,HCO2 (INCLUDING CALCULATED O2 SATURATION) BLUE CROSS BLUE SHIELD HEALTHCARE PLAN OF GEORGIA, INC. IN HOUSE LAB LIST EFFECTIVE 06/01/13 * 1 CPT DESCRIPTION 82805 GASES, BLOOD, ANY COMBINATION OF PH,PCO2,PO2,CO2,HCO2(INC CALCULATED O2 SATURATION BY DIRECT MEASUREMENT, EXCEPT PULSE OXIMETRY 82945 GLUCOSE; BODY FLUID OTHER THAN BLOOD 82947 GLUCOSE; QUANTITATIVE, BLOOD (EXCEPT REAGENT STRIP) 82948 GLUCOSE; BLOOD, REAGENT STRIP 83002 GONADOTROPHIN; LH 83014 HELICOBACTER PYLORI BREATH TEST ANALYSIS FOR UREASE ACTIVITY; DRUG ADMINISTRATION 83036 HEMOGLOBIN; GLYCATED 83518 IMMUNOASSAY, FOR ANALYTE OTHER THAN ANTIBODY OR INFECTIOUS AGENT ANTIGEN; SINGLE STEP METHOD 83661 FETAL LUNG MATURITY ASSESSMENT; (L/W) RATIO 83861 TEAR OSMOLARITY 84081 PHOSPHATIDYLGLYCEROL 84132 POTASSIUM; SERUM 84144 PROGESTERONE 84157 PROTEIN, OTHER SOURCE (eg SYNOVIAL FLUID, CEREBROSPINAL FLUID 84484 TROPONIN, QUANTITATIVE 84520 UREA NITROGEN (BUN); QUANTITATIVE 84703 GONADOTROPIN, CHORIONIC (hCG): QUALITATIVE 85002 BLEEDING TIME 85004 BLOOD COUNT; AUTOMATED DIFFERENTIAL WBC COUNT 85007 BLOOD COUNT;BLOOD SMEAR MICROSCOPIC EXAMINATION WITH MANUAL DIFFERENTIALWBC 85008 BLOOD COUNT; BLOOD SMEAR MICROSCOPIC EXAMINATION WITHOUT MANUAL DIFFERENTIAL SBC COUNT 85009 BLOOD COUNT; MANUAL DIFFERENTIAL WBC COUNT, BUFFY COAT 85013 BLOOD COUNT;SPUN MICROHEMATOCRIT 85014 BLOOD COUNT; HEMATOCRIT (HCT) 85018 BLOOD COUNT; HEMOGLOBIN (HGB) 85025 BLOOD COUNT;COMPLETE (CBC),AUTOMATED (HGB, HCT, RBC, WBC AND PLATELET COUNT) AND AUTOMATED DIFFERENTIAL WBC 85027 BLOOD COUNT;COMPLETE (CBC), AUTOMATED (HGB,HCT,RBC,WBC AND PLATELET COUNT) 85041 BLOOD COUNT; RED BLOOD CELL (RBC) AUTOMATED 85044 BLOOD COUNT; RETICULOCYTE, MANUAL 85045 BLOOD COUNT; RETICULOCYTE, AUTOMATED BLOOD COUNT; LEUKOCYTE, AUTOMATED 85048 85097 BONE MARROW, SMEAR INTERPRETATION 85379 D-DIMER 85460 HEMOGLOBIN OR RBCS, FETAL, FOR FETOMATERNAL HEMORRHAGE; DIFFERENTIAL 85461 HEMOGLOBIN OR RBCS, FETAL, FOR FETOMATERNAL HEMORRHAGE; ROSETTE 85576 PLATELET; AGGREGATION (IN VITRO), EACH AGENT 85610 PROTHROMBIN TIME BLUE CROSS BLUE SHIELD HEALTHCARE PLAN OF GEORGIA, INC. IN HOUSE LAB LIST EFFECTIVE 06/01/13 * * * 2 CPT DESCRIPTION 85651 SEDIMENTATION RATE, ERYTHROCYTE; NON-AUTOMATED 85652 SEDIMENTATION RATE, ERYTHROCYTE; AUTOMATED THROMBOPLASTIN TIME, PARTIAL (PTT); PLASMA OR WHOLE BLOOD 85730 86077 BLOOD BANK PHYSICIAN SERVICES 86140 C-REACTIVE PROTEIN 86308 HETEROPHILE ANTIBODIES; SCREENING 86403 PARTICLE AGGLUTINATION; SCREEN, EACH ANTIBODY 86406 PARTICLE AGGLUTINATION; TITER, EACH ANTIBODY 86580 SKIN TEST; TUBERCULOSIS, PATCH OR INTRADERMAL 86756 RESPIRATORY SYNCYTIAL VIRUS 86759 ANTIBODY; ROTAVIRUS 86850 ANTIBODY SCREEN, RBC, EACH SERUM TECHNIQUE 86880 ANTIHUMAN GLOBULIN TEST(COOMBS TEST) ;DIRECT, EACH ANTISERUM 86885 ANTIBODY GLOBULIN (COOMBS), INDIRECT 86900 BLOOD TYPING, ABO 86901 BLOOD TYPING; RH(D) 86920 COMPATIBILITY TEST; EACH UNIT; IMMEDIATE SPIN TECHNIQUE 86921 COMPATIBILITY TEST; EACH UNIT; INCUBATION TECHNIQUE 86922 COMPATIBILITY TEST; EACH UNIT; ANTIGLOBULIN TECHNIQUE 86923 COMPATIBILITY TEST; EACH UNIT, ELECTRONIC 87045 CULTURE, BACTERIAL; STOOL, AEROBIC WITH ISOLATION AND PRELIMINARY EXAM 87110 CULTURE; CHLAMYDIA, ANY SOURCE 87164 DARKFIELD EXAMINATION, ANY SOURCE (EG., PENILE, VAGINAL, ORAL,SKIN); IN-CLUDES 87205 SMEAR, PRIMARY SOURCE, WITH INTERPRETATION; GRAM STAIN OR GIEMSA STAIN 87210 SMEAR PRIMARY SOURCE WITH INTERPRETATION; WET MOUNT FOR INFECT AGENTS 87220 TISSUE EXAMINATION FOR FUNGI OF SAMPLES FROM SKIN, HAIR, OR NAILS FOR FUNGI OR ECTOPARASITE 87280 INFECTIOUS AGENT DETECTION BY DIRECT FLOURESCENT ANTIBODY TECHNIQUE 87400 INFECTIOUS AGENT ANTIGEN DETECTION BY ENZYME IMMUNOASSAY TECH,QUAL/SEMI-QUANTI 87803 C-DIFF TOXIN 87807 INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOASSAY WITH DIRECT OPTICAL OBSERVATION; INFLUENZA INFECTIOUS AGENT ANTIGEN DETECTION BY IMMUNOASSAY WITH DIRECT OPTICAL OBSERVATION; RESPIRATORY SYNCYTIAL VIRUS 87880 INFECTIOUS AGENT DETECTION BY IMMUNOASSAY WITH DIRECT OPTICAL OBSERVATION; STREPTOCOCCUS GRP A 87905 INFECTIOUS AGENT ENZYMATIC ACTIVITY OTHER THAN VIRUS (eg. SIALIDASE ACTIVITY IN VAGINAL FLUID) 88172 CYTOPATHOLOGY; EVALUATION OF FINE NEEDLE ASPIRATE; IMMEDIATE 88173 CYTOPATHOLOGY; EVALUATION OF FINE NEEDLE ASPIRATE; FINAL INTERPRETATATION & REPORT 88319 GROUP III FOR ENZYME CONSTITUENTS (WBC STAIN) 87804 BLUE CROSS BLUE SHIELD HEALTHCARE PLAN OF GEORGIA, INC. IN HOUSE LAB LIST EFFECTIVE 06/01/13 * * * 3 88738 HEMOGLOBIN (HgB), QUANTITATIVE, TRANSCUTANEOUS 89050 CELL COUNT, MISCELLANEOUS BODY FLUIDS (E.G. CSF, JOINT FLUID), EXCEPT BLOOD CPT DESCRIPTION 89051 CELL COUNT, MISCELLANEOUS BODY FLUIDS (E.G. CSF, JOINT FLUID), EXCEPT BLOOD WITH DIFFERENTIAL COUNT 89060 CRYSTAL IDENTIFICATION BY LIGHT MICROSCOPY WITH OR WITHOUT POLARIZING LENS ANALYSIS 89230 SWEAT COLLECTION BY IONTOPHORESIS 89250 CULTURE AND FERTILIZATION 89253 ASSISTED EMBRYO HATCHING, MICROTECHNIQUES 89254 OOCYTE ID FROM FOLLICULAR FLUID 89255 PREP OF EMBRYO FOR TRANSFER (ANY METHOD) 89258 CRYOPRESERVATION; EMBRYO (S) 89259 CRYOPRESERVATION; SPERM 89260 SPERM ISOLATION; SIMPLE PREP 89272 EXTENDED CULTURE OF OOCYTES/EMBRYOS 4-7 DAYS 89280 ASSISTED OOCYTE FERTILIZATION,MIC 89281 ASSISTED OOCYTE FERTILIZATION,MIC > 10 OOCYTES 89290 BIPOSY, OOCYTE POLAR BODY OR EMBRYO BLASTOMERE 89291 BIOPSY, OOCYTE POLAR BODY OR EMBRYO BLASTOMERE> 5 EMBRYOS 89300 SEMEN ANALYSIS; PRESENCE AND/OR MOTILITY OF SPERM INCLUDING HUHNER TEST (POST COITAL) 89310 89320 SEMEN ANALYSIS; MOTILITY & COUNT (NOT INCLUDING HUHNER TEST) SEMEN ANALYSIS; COMPLETE (VOLUME, COUNT, MOTILITY AND DIFFERENTIAL) 89321 SEMEN ANALYSIS, PRESENCE AND/OR MOTILITY OF SPERM 89322 SEMEN ANALYSIS; VOLUME, COUNT MOTILITY AND DIFFERENTIAL USING STRICT MORPHOLOGIC CRITERIA (E.G. KRUGER) 89325 SPERM ANTIBODIES 89329 SPERM EVALUATION; HAMSTER PENETRATION 89330 SPERM EVALUATION;CERVICAL MUCUS PENETRATION TEST, WITH OR WITHOUT SPINNBARKEIT TEST 89331 SEMEN EVALUATION: FOR RETROGRADE EJACULATION, URINE (SPERM 89335 CRYOPRESERVATION, REPRODUCTIVE TISSUE 89342 STORAGE, (PER YEAR); EMBRYO(S) 89343 STORAGE; SPERM/SEMEN 89346 STORAGE, (PER YEAR); OOCYTE(S) 89352 THAWING OF CRYOPRESERVED; EMBRYO(S) 89356 THAWING OF CRYOPRESERVED; OOCYTES * * * * * * * * * * * * * * * * * * * * * = added 11/15/2012 BLUE CROSS BLUE SHIELD HEALTHCARE PLAN OF GEORGIA, INC. IN HOUSE LAB LIST EFFECTIVE 06/01/13 4






