SmithSupplement.
advertisement

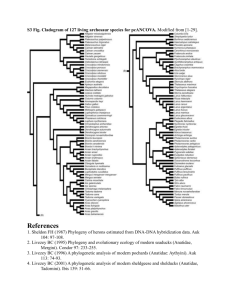
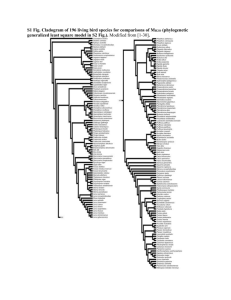
Smith-supplement1 Supplementary data associated with: Evolution of body mass in the Pan-Alcidae (Aves, 2 Charadriiformes): the effects of combining neontological and paleontological data 3 N. Adam Smith1, 2, 3* 1 National Evolutionary Synthesis Center 2024 W. Main St., Suite A200, Durham, NC, USA; 2 3 4 North Carolina Museum of Natural Sciences, 11 W. Jones St., Raleigh, NC, USA; The Field Museum of Natural History, 1400 South Lake Shore Drive, Chicago, IL, USA *correspondence: adam_smith@utexas.edu 1 Smith-supplement5 Supplementary Appendix 1 6 Measurements of Sampled Taxa 7 2 Data Sources.—Body mass data for extant alcids are from Dunning (2008), with the 8 exception of C. carbo, which is from Gaston and Jones (1998). Body length data for extant alcids 9 are from del Hoyo et al. (1996). Measurement data for extant alcid eggs were assembled from the 10 following sources: (Baicich and Harrison 1997, Davie 1900, De Santo and Nelson 1995, del 11 Hoyo et al. 1996, Friesen et al. 1996, Gaston and Jones 1998, Hipfner et al. 2010, Hunter et al. 12 2002, Kaler et al. 2009, Konyukhov and Kitaysky 1995, Livezey 1988, Nelson and Hamer 1995). 13 Measurements of specimens representing extinct taxa were taken directly from holotype and 14 referred specimens. Skeletal measurements follow von den Driesch (1976), were taken using 15 digital calipers and were rounded to the nearest 0.1 millimeter. All masses are stated in grams (g) 16 and all length, breadth and width measurements are stated in millimeters (mm). With respect to 17 skeletal specimens representing extant species, a total of five or more adult specimens of each 18 species, assessed based upon degree of ossification (Chapman 1965), and including both sexes, 19 were evaluated whenever available. Skeletal specimens from multiple locations within the 20 geographic range of extant species (i.e., subspecies) were examined to account for potential 21 geographic variation within species. Sexual dimorphism in extant Alcidae is not significant with 22 respect to many life history traits, including plumage and body mass in some species (e.g., 23 Common Murre Uria aalge; (Nettleship and Birkhead 1985, Storer 1952, Székely et al. 2000). 24 Measurement data for extant alcid eggs were assembled from the following sources: (Baicich 25 and Harrison 1997, Davie 1900, De Santo and Nelson 1995, del Hoyo et al. 1996, Friesen et al. 26 1996, Gaston and Jones 1998, Hipfner et al. 2010, Hunter et al. 2002, Kaler et al. 2009, 27 Konyukhov and Kitaysky 1995, Livezey 1988, Nelson and Hamer 1995). Additional oological Smith-supplement- 3 28 data was collected from specimens housed in the collection of the Smithsonian National Museum 29 of Natural History (USNM). Body length data used for extant alcids are those compiled by del 30 Hoyo et al. (1996). 31 Associated skeletons of the Great Auk are not known (Fuller 1987, Livezey 1988). 32 Therefore, measurement data representing that species was collected from composite skeletons 33 and additional material from a series of disarticulated remains in the USNM collection that were 34 collected during the Lucas expedition to Funk Island (Lucas 1890). Despite numerous accounts 35 regarding the natural history of the Great Auk, only a single measurement of the mass of the 36 species is known. Feilden (1872) reported an individual weighing 9 Danish pounds, the 37 equivalent of ~4,500 g. The mass of the Great Auk has twice been independently estimated at 38 ~5,000 g using a variety of bodily and oological dimensions (Coues 1868, Livezey 1988). An 39 intermediate value of 4,750 g is used herein. As with the lack of data on the body mass of the 40 Great Auk, measurements of fresh Great Auk eggs are not known. However, egg dimensions and 41 egg mass are strongly correlated across Aves (Anderson et al. 1979) and a previously generated 42 estimate of 327 g is used herein (Birkhead 1993). Additionally, newly collected measurements of 43 Great Auk body length and oological dimensions were included in an attempt to independently 44 estimate its body mass. The forelimbs (i.e., humerus, radius, ulna, and carpometacarpus) of 45 flightless pan-alcids (i.e., †Pinguinus and †Mancallinae) are relatively shorter than those of 46 volant pan-alcids (Howard 1971, Livezey 1988, Owen 1864, Raikow et al. 1988). Therefore, 47 non-forelimb skeletal variables (e.g., femoral dimensions) were evaluated to identify an 48 appropriate variable for estimating the body mass of flightless species. Measurements of the 49 mandible, vertebrae and pelvis were not evaluated because of the relative rarity of those elements 50 representing the flightless †Mancallinae lineage (Smith 2011b). Smith-supplement51 4 Institutional Abbreviations.—ANSP— Academy of Natural Sciences of Philadelphia, PA, 52 USA; GCVP—Georgia College Vertebrate Paleontology Collection, Milledgeville, GA, USA; 53 LACM—Natural History Museum of Los Angeles County, Los Angeles, CA., USA; NCSM— 54 North Carolina Museum of Natural Sciences, Raleigh, North Carolina, USA; SDSNH—San 55 Diego Natural History Museum, San Diego, CA, USA; UMMZ—University of Michigan 56 Museum of Zoology, Ann Arbor, MI, USA; UF/PB—Florida Museum of Natural History/Pierce 57 Brodkorb Collection, Gainesville, FL, USA; USNM—National Museum of Natural History, 58 Smithsonian Institution, Washington, D.C., USA. 59 60 Anatomical Abbreviations.—BM, body mass; BL, body length; EM, egg mass; EL, egg 61 length; ED, egg diameter; gbS. greatest breadth of skull; ghS, greatest height of skull; mlSt, 62 maximum length of sternum; dlSt, dorsal length of sternum; lcSt, length of sternal carina; sbRF, 63 smallest breadth between costal rib facets (on sternum); glC, greatest length of coracoid; mlC, 64 medial length of coracoid; bbC, basal breadth of coracoid; bfC, breadth of facies articularis 65 basalis of coracoid; diSc, diagonal of scapula; glH, greatest length of humerus; bpH, breadth of 66 proximal humerus; dpH, depth of proximal humerus; swH, shaft width of Humerus (at 67 midpoint); bdH, breadth of distal humerus; ddH, depth of distal humerus; glR, greatest length of 68 radius; bpR, breadth of proximal radius; swR = greatest width of radial shaft at midpoint; bdR, 69 breadth of distal radius; glU, greatest length of ulna; bpU, breadth of proximal ulna; swU, width 70 of ulnar shaft; bdU, breadth of distal ulna; ddU, diagonal of distal ulna; glF, greatest length of 71 femur; mlF, medial length of femur; bpF, breadth of proximal femur; dpF, depth of proximal 72 femur; swF, width of femoral shaft; bdF, breadth of distal femur; ddF, depth of distal femur; glT, 73 greatest length of tibiotarsus; laT, axial length of tibiotarsus; dpT, diagonal of proximal Smith-supplement- 5 74 tibiotarsus; swT, width of tibial shaft; bdT, breadth of distal tibiotarsus; ddT, depth of distal 75 tibiotarsus; glTm, greatest length of tarsometatarsus; bpTm, breadth of proximal tarsometatarsus; 76 swTm, width of tarsometatarsal shaft; bdTm, breadth of distal tarsometatarsus. 77 78 79 Measurement Data.—See supplementary file "Appendix_1.xlsx" for raw values for all sampled taxa. Dyrad.org DOI: 10.5061/dryad.3k7v7. Smith-supplement80 Supplementary Appendix 2 81 82 83 84 Avian Body Mass Ranges for 65 Avian Clades 6 Values for clades with ranges exceeding that of Pan-Alcidae are bolded. Taxon Alcidae (n = 34) (extant alcids) Alcidae (n = 35) (including P. impennis) Pan-Alcidae (n = 60) (pan-alcids) Stercorariidae (n = 16) (skuas) Laridae (n = 99) (gulls) Sternidae (n = 60) (terns) Turnicidae (n = 24) (buttonquail) Scolopacidae (n = 154) (snipes and sandpipers) Jacanidae (n = 13) (jacanas) Haematopididae (oystercatchers)(n = 15) Recurvirostridae (stilts) (n = 13) Burhinidae (n = 10) (thick-knees) Glareolidae (n = 16) (coursers & pratincoles) Charadriidae (n = 77) (plovers & lapwings) Spheniscidae (n = 40) (penguins) Gaviidae (n = 7) (loons) Podicipedidae (n = 45) (grebes) Diomedeidae (n = 40) (albatross) Procellariidae (n = 98) (petrels & shearwaters) Hydrobatidae (n = 30) (storm petrels) Minimum (g) 84 (Aethia pusilla) 84 (Aethia pusilla) 84 (Aethia pusilla) 270 (Stercorarius longicaudus) 118 (Larus minutus) 42 (Sternula saundersi) 18 (Ortyxelos meiffrenii) 21 (Caladris minuta) 41 (Microparra capensis) 517 (Haematopus finschi) 161 (Himantopus himantopus) 320 (Burhinus vermiculatus) 37 (Glareola cinerea) 12 (Charadrius thoracicus) 842 (Eudyptula minor) 1486 (Gavia stellata) 116 (Tachybaptus dominicus) 2,060 (Thalassarche chlororhynchos) 99 (Bulweria bulwerii) 17 (Oceanites gracilis) Maximum (g) 992 (Uria aalge) 4750 (Pinguinus impennis) 5,363 (Miomancalla howardae) 1,935 (Stercorarius antarcticus) 1,855 (Larus hyperboreus) 655 (Hydroprogne caspia) 110 (Turnix ocellatus) 869 (Numenius arquata) 261 (Actophilornis albinucha) 819 (Haematopus fuliginosus) 361 (Recurvirostra andina) 1032 (Esacus magnirostris) 150 (Rhinoptilus chalcopterus) 387 (Vanellus miles) 38,200 (Aptenodytes forsteri) 5,460 (Gavia immer) 1,646 (Podiceps major) 10,300 (Diomedea epomorpha) 4,940 (Macronectes giganteus) 86 (Oceanodroma tristrami) Range (g) 908 4,666 5,279 1,665 1,737 613 92 848 220 302 200 712 113 375 37,358 3,974 1,530 8,240 4,841 69 Smith-supplementPelecanoididae (n = 6) (diving petrels) Pelecanidae (n = 14) (pelicans) Sulidae (n = 30) (gannets & boobies) Phalacrocoracidae (n = 79)(cormorants) Anhingidae (n = 6) (darters) Ardeidae (n = 78) (herons) Ciconiidae (n = 21) (storks) Threskiornithidae (ibis) (n = 32) Anatidae (n = 320) (ducks & geese) Cathartidae (n = 12) (vultures) Accipitridae (n = 348) (hawks & eagles) Falconidae (n = 116) (falcons) Megapodiidae (n = 29) (mound-builders) Cracidae (n = 58) (curassows & guans) Pteroclidae (n = 12) (sandgrouse) Tetraonidae (n = 43) (grouse) Odontophoridae (quail) (n = 47) Phasianidae (n = 180) (pheasants) Gruidae (n = 27) (cranes) Rallidae (n = 173) (rails & crakes) Otididae (n = 30) (bustards) Apodidae (n = 105) (swifts) Trochilidae (n = 372) (hummingbirds) Trogonidae (n = 38) (trogons) Alcedinidae (n = 94) (kingfishers) 121 (Pelecanoides georgicus) 3,174 (Pelecanus occidentalis) 857 (Sula sula) 427 (Phalacrocorax niger) 1235 (Anhinga anhinga) 80 (Ixobrychus involucris) 1,081 (Anastomus lamelligerus) 511 (Plegadis ridgwayi) 266 (Nettapus auritus) 935 (Cathartes burrovianus) 74 (Accipiter superciliosus) 41 (Microhierax latifrons) 308 (Megapodius laperouse) 439 (Ortalis leucogastra) 160 (Pterocles quadricinctus) 257 (Bonasa sewerzowi) 115 (Colinus leucopogon) 45 (Coturnix adansonii) 2,417 (Anthropoides virgo) 24 (Porzana flaviventer) 585 (Eupodotis senegalensis) 5 (Collocalia esculenta) 2 (Thaumastura cora) 42 (Trogon violaceus) 10 (Ispidina lecontei) 239 (Pelecanoides garnotii) 11,450 (Pelecanus onocrotalus) 3,067 (Morus capensis) 3,500 (Phalacrocorax harrisi 1,700 (Anhinga melanogaster) 4,468 (Ardea goliath) 6,892 (Jabiru mycteria) 3,515 (Pseudibis gigantea) 11,900 (Cygnus buccinator) 12,500 (Vultur gryphus) 8177 (Gyps coprotheres) 1,752 (Falco rusticolus) 2,520 (Alectura lathami) 4133 (Crax rubra) 428 (Pterocles orientalis) 4,100 (Tetrao urogallus) 457 (Odontophorus capueira) 4,766 (Pavo cristatus) 8,786 (Grus japonensis) 2,700 (Fulica gigantea) 11,975 (Otis tarda) 180 (Hirundapus celebensis) 20 (Patagona gigas) 206 (Pharomachrus mocinno) 356 (Dacelo novaeguineae) 7 118 8,276 2,210 3,073 465 4,388 5,811 3,004 11,634 11,565 8,103 1,711 2,212 3,694 268 3,843 342 4,721 6,369 2,676 11,390 175 18 164 346 Smith-supplementMeropidae (n = 21) (bee eaters) Coraciidae (n = 12) (rollers) Bucerotidae (n = 83) (hornbills) Galbulidae (n = 12) (jacamars) Bucconidae (n = 29) (puffbirds) Capitonidae (n = 94) (barbets) Ramphastidae (n = 68) (toucans & aracaris) Indicatoridae (n = 24) (honeyguides) Picidae (n = 247) (woodpeckers) Columbidae (n = 296) (doves & pigeons) Cacatuidae (n = 29) (cockatoos) Psittacidae (n = 321) (parrots) Musophagidae (n = 23) (turacos) Cuculidae (n = 189) (cuckoos & allies) Tytonidae (n = 15) (barn owls) Strigidae (n = 249) (true owls) Caprimulgidae (n = 84) (nightjars & allies) Passeriformes (n = 6,593) (oscines & suboscines) Cinclidae (n = 5) (dippers) Tinamidae (n = 59) (tinamous) 15 (Merops orientalis) 96 (Eurystomas gularis) 97 (Tockus camurus) 16 (Brachygalba lugubris) 16 (Nonnula frontalis) 9 (Pogoniulus simplex) 121 (Pteroglossus inscriptus) 10 (Prodotiscus zambesiae) 9 (Picumnus aurifrons) 29 (Columbina passerina) 92 (Nymphicus hollandicus) 12 (Loriculus tener) 198 (Corythaixoides leucogaster) 18 (Chrysococcyx meyeri) 195 (Phodilus prigoginei) 41 (Micrathene whitneyi) 25 (Nyctiphrynus yucatanicus) 4 (Phylloscopus sichuanensis) 37 (Cinclus shulzi) 43 (Taoniscus nanus) 60 (Merops nubicoides) 171 (Coracias temminckii) 4,191 (Bucorvus leadbeateri) 63 (Jacamerops aureus) 106 (Monasa morphoeus) 202 (Megalaima virens) 709 (Ramphastos swainsonii) 54 (Melichneutes robustus) 516 (Campephilus principalis) 2,384 (Goura victoria) 841 (Probosciger aterrimus) 2000 (Strigops habroptila) 965 (Corythaeola cristata) 769 (Centropus milo) 887 (Tyto multipunctata) 2,992 (Bubo bubo) 174 (Eurostopodus mystacalis) 1,135 (Corvus crassirostris) 88 (Cinclus pallasii) 1,636 (Tinamus solitarius) 8 45 75 4,094 47 90 193 588 44 507 2,355 749 1,988 767 751 692 2,951 149 1,132 51 1,593 85 86 Supplementary Appendix 2 Footnote: Number of taxa sampled in each clade (excluding Pan- 87 Alcidae which includes extinct taxa) reflects the number of data points provided by Dunning 88 (2008; i.e., includes males, females and sub-species). Taxonomy follows Dunning (2008) and all 89 values are means from Dunning (2008) rounded to nearest 1.0 gram. Despite small sample sizes, Smith-supplement- 9 90 Gaviidae (n = 7), Pelecanoididae (n = 6), Pelecanidae (n = 14) and Anhingidae (n = 6) were 91 included to sample as many diving taxa as possible. Despite small sample sizes, clades such as 92 Burhinidae (n = 10) and Jacanidae (n = 13) were sampled to include as broad a range of 93 charadriiforms as possible. Based on previous phylogenetic results (Baker et al., 2007), 94 Ptercolidae is not considered a part of Charadriiformes herein. The following taxa were not 95 included owing to small sample sizes (i.e., relatively few extant species in comparison with 96 Alcidae): Struthionidae; Rheidae; Casuariidae; Dromaiidae; Apterygidae; Phaethontidae; 97 Fregatidae; Scopidae; Balaenicipididae, Phoenicopteridae, Anhimidae; Pandionidae; 98 Sagittariidae; Meleagrididae; Numididae; Opisthocomidae; Mesitornithidae, Aramidae; 99 Psophiidae; Heliornithidae; Rhynochetidae; Eurypygidae; Cariamidae; Hemiprocnidae; Coliidae; 100 Todidae; Momotidae; Brachypteraciidae; Leptosomatidae; Upupidae; Phoeniculidae; 101 Rostratulidae; Dromadidae; Ibiorhynchidae; Pluvianellidae; Pedionomidae; Thincoridae; 102 Chionidae; Rynchopidae; Steatornithidae; Podargidae; Nyctibiidae. Smith-supplement103 104 Supplementary Figure 1. Bar graph of avian body mass ranges. 10 Smith-supplement105 11 Supplementary Appendix 3 106 107 108 Supplementary Body Mass Estimation Methods and Results Additional Details of Body Mass Estimation for Flightless Pan-Alcidae.—As stated above, 109 forelimb measurements of the Great Auk were excluded from initial PGLS analyses because of 110 the markedly different allometric relationship between those skeletal elements and body mass in 111 flightless pan-alcids (i.e., flightless pan-alcids have relatively small wings). Attempts to 112 independently estimate the body mass of the Great Auk were not successful. No individual, non- 113 forelimb variable met all three criteria (see methods) evaluated to assess the predictive power of 114 variables in relation to body mass. This included explorations of non-skeletal variables (e.g., 115 body length, egg volume). Therefore, as stated above, a conservative body mass estimate of 116 4,750 g was used for the Great Auk. The body mass estimate for the Pliocene sister taxon of the 117 Great Auk, †Pinguinus alfrednewtoni (Olson 1977), was generated by assessing the ratio of body 118 mass to humeral length in the Great Auk (4,750 g: 104.1 mm = 45.63 g/mm) and extrapolating to 119 produce an estimate for †P. alfrednewtoni (101.0 mm x 45.63 g/mm = 4,609 g; Table 2). This 120 estimate is consistent with the overall smaller size of †P. alfrednewtoni relative to †P. impennis 121 (based on greatest length of coracoids, humeri and femora; see Appendix 1). If the body mass of 122 †P. impennis is assumed to be 5,000 g (sensu Bédard 1969, Birkhead 1993, Coues 1868, Livezey 123 1988), then the estimate for †P. alfrednewtoni increases to 4851 g, a difference of only 242 g 124 (i.e., < 5.3 %). 125 Estimation of body mass for species of the flightless †Mancallinae clade (Lucas Auks) 126 was complicated owing to the lack of complete skeletons representing the 6 included species. 127 Moreover, as in †Pinguinus, the forelimbs of Lucas auks are relatively small in comparison to Smith-supplement- 12 128 their overall body mass, putatively based on the relative dimensions of other skeletal elements 129 (Howard 1970). The best non-forelimb predictor of body mass among extant alcids (including 130 the Great Auk) was the greatest length of the femur (glF; r2 = 0.95, AICc = -62.1, λ = 0.9; Fig. 131 1B; Table 1). Femur length was also identified as a reliable predictor of body mass in a larger 132 sample of charadriiforms [(Field et al. 2013), figure 6, r2 = 0.935)]. Although femoral length is 133 potentially linked with phylogeny (λ = 0.9) and the percent predicted error associated with 134 estimates based on this regression is relatively high in some instances (PPE: range = 1.1-66.9%; 135 average = 25.3%; median = 22.9%), more precise estimates will only be possible as additional 136 fossil material is referred to the species level in †Mancallinae (i.e., specimens that would 137 facilitate a multi-step regression). Comparisons with previous estimates for these taxa are not 138 feasible given the methodological issues with the only previous analysis of body mass of 139 mancalline auks (Livezey, 1988; discussed below). Estimates for †Miomancalla howardae, 140 †Mancalla cedrosensis and †Mancalla lucasi were generated using femoral measurements from 141 the holotypes of those species. However, femora representing †Miomancalla wetmorei, 142 †Mancalla vegrandis and †Mancalla californiensis are not known (Smith 2011b). Therefore, to 143 derive a body mass estimate for those †Mancallinae species lacking femora, the ratio of humerus 144 length to femoral length in †M. howardae (0.774), †M. cedrosensis (0.779), and †M. lucasi 145 (0.752) was calculated and then the average ratio (glH:glF = 0.74) was used to estimate femoral 146 lengths for †M. wetmorei, †M. vegrandis and †M. californiensis (Table 2). Body mass estimates 147 for †M. wetmorei, †M. vegrandis and †M. californiensis were then generated using the same 148 regression equation based on the relationship between greatest length of the femur and body 149 mass in extant alcids (Table 2). 150 Smith-supplement151 13 Comparisons with Previous Studies.—Previous body mass estimates for extinct pan- 152 alcids are restricted to a single volant species, the large Pliocene auk †Alca stewarti and some 153 flightless species including the Great Auk and selected members of the Alcidae stem lineage 154 Mancallinae (Lucas Auks; Livezey 1988, Martin et al. 2001). Body length was not strongly 155 correlated with body mass regardless of whether the Great Auk was included (Table 1) or 156 excluded (r2 = 0.86, AICc = -29.9, λ = 0.0). In contrast, body length contributed significantly to 157 the multi-step regression used by Livezey (1988) to estimate a 4,999 g body mass for the Great 158 Auk. However, Livezey (1988) also included measures of culmen length, which varies with diet 159 (relatively shorter beaks in planktivorous species versus longer beaks in piscivorous species) and 160 tail length. Tail length variability in Alcidae has not been studied in detail. However, differences 161 in locomotor style (both underwater and aerial) and incubation posture (i.e., upright as in 162 Cepphus versus pronated as in Alca; see Birkhead 1993, figure 9) are likely factors influencing 163 variability in alcid tail length. Body length may prove to be a better estimator in avian clades 164 other than Alcidae, clades with more homogeneous ecologies and ethologies. 165 Flightless pan-alcids and penguins possess pachyostotic limb bones that are relatively 166 greater in mass than those of comparably sized volant pan-alcids (Ksepka 2007, Smith and 167 Clarke 2014). However, previous comparison of body mass with skeletal mass across a broad 168 sample of Aves did not identify penguins as an outlier and skeletal mass is a minor contributor to 169 overall mass in birds (Prange et al. 1979). Therefore, while it remains possible that body mass 170 estimates based on regressions generated using data from extant, volant alcids with non- 171 pachyostotic limb bones may underestimate the mass of flightless pan-alcids, it seems unlikely 172 that pachyostotic limb bones would introduce significant bias into these estimates. Smith-supplement- 14 173 It is not surprising that egg mass, egg length and egg diameter were not strongly 174 correlated with body mass in Alcidae as Charadriiformes in general, and alcids in particular, are 175 characterized by a wider range of developmental strategies than most other avian clades (i.e., 176 semi-precocial, intermediate, precoccial (De Santo and Nelson 1995). Developmental strategy 177 has been correlated with a variety of life history traits including egg mass and associated egg 178 dimensions (Ricklefs and Starck 1998). Exclusion of potentially inaccurate estimates for the 179 body mass and egg mass of the Great Auk did not result in stronger support for these non- 180 skeletal variables as good predictors of body mass in Alcidae (results not shown). 181 Body mass estimates for extinct pan-alcids were previously restricted to †A. stewarti, †P. 182 impennis and some species of †Mancallinae (Livezey, 1988; Martin et al., 2001). The previously 183 generated ~1,900 g estimate for the large volant auk A. stewarti (Dyke and Walker, 2001) is 184 relatively consistent with the ~2,100 g estimate generated herein (Table 2). However, further 185 comparisons with previous studies that estimated the body mass of pan-alcids are limited 186 because those previous studies (Livezey, 1988; Martin et al., 2001) did not report the 187 measurement data on which their regressions and resulting estimates were based, did not provide 188 the catalog numbers of specimens from which measurements were taken, and did not consider 189 the potentially biasing effect of phylogeny (Harvey and Pagel, 1991). Moreover, both previous 190 studies that estimated extinct pan-alcid body masses (Livezey, 1988; Martin et al., 2001) relied 191 on measurement data from fossil specimens that were previously attributed to species on the 192 basis of size or provenance, and that may not have been representative of the taxa they were 193 referred to. For example, both previous studies reportedly considered measurement data for 194 humeri and ulnae of †Mancalla milleri. Martin et al. (2001) utilized measurements reported in 195 the original description of M. milleri (Howard, 1970). In contrast, no sources of data or specimen Smith-supplement- 15 196 numbers were reported by Livezey (1988), and it is unclear from what specimens data were 197 collected. Furthermore, the holotype specimen of †M. milleri is a femur (LACM 2185) and 198 associated specimens that would allow for referral of elements other than femora to that taxon 199 are not known (Smith 2011b). Moreover, the holotype specimen of †M. milleri is now 200 considered Pan-Alcidae incertae sedis and some material in the size range of †M. milleri is now 201 referred to †Mancalla vegrandis (Smith, 2011b). Data reported for †Mancalla cedrosensis, 202 †Mancalla emlongi and †Mancalla diegensis by Livezey (1988) also lack documentation of 203 specimen numbers or citation of sources and include elements not comparable to the holotypes of 204 those taxa. Finally, it is unclear what specimens Livezey (1988) included in '†Praemancalla spp.'. 205 The lack of documentation, repeatability and phylogenetic context, in combination with the 206 inclusion of specimens of uncertain taxonomic referral call into question the reliability of those 207 previous estimates. 208 Smith-supplement- 16 209 Supplementary Table A3.1: Mean and 95% confidence interval of maximum likelihood 210 estimates of ancestral body mass for nodes in extant Alcidae (lettered nodes correspond to those 211 in Figure 3). Node A B C D E F G H I J K L M N O P Q R S T U V 212 213 Estimate 415.31 411.94 222.71 194.53 200.57 208.06 550.86 627.11 607.58 421.17 434.66 442.64 538.70 556.71 868.81 236.85 218.88 167.05 462.08 549.25 325.79 269.74 Lower CI 208.03 204.03 56.90 59.98 73.42 80.33 371.09 482.96 497.38 243.12 261.14 267.70 356.42 370.51 710.78 62.12 76.96 87.13 298.44 429.42 122.45 91.70 Upper CI 622.60 619.84 388.53 329.07 327.71 335.79 730.64 771.25 717.78 599.22 608.18 617.57 720.98 742.92 1026.84 411.58 360.80 246.98 625.73 669.07 529.12 447.79 Smith-supplement- 17 214 Supplementary Table A3.2: Mean and 95% confidence interval of maximum likelihood 215 estimates of ancestral body mass for nodes in Pan-Alcidae (lettered nodes correspond to those in 216 Figure 2). Node A B C D E F G H I J K L M N O P Q R S T U V W X Y Z a b c d e f g h i j k l m Estimate 1236.50 1041.71 966.58 994.78 1106.61 1382.39 1809.68 1678.35 1672.33 1613.24 1515.77 1374.35 850.95 4399.68 817.67 925.18 960.39 558.66 287.65 570.62 346.48 240.01 180.17 236.74 607.08 544.68 541.42 544.14 393.53 331.87 282.94 259.65 486.91 359.49 280.42 227.21 224.82 198.78 501.74 LowerCI 1136.67 955.44 886.93 919.63 1034.54 1313.38 1743.06 1617.313 1610.374 1551.747 1457.527 1322.036 808.6849 4354.418 759.4156 872.8599 918.1272 508.3691 245.6618 528.6617 283.5357 186.57 137.75 192.78 536.85 483.76 488.47 501.79 322.35 270.65 229.92 217.29 414.87 291.41 216.53 168.26 172.34 156.49 438.20 UpperCI 1336.33 1127.97 1046.22 1069.94 1178.68 1451.40 1876.31 1739.39 1734.29 1674.74 1574.01 1426.67 893.21 4444.96 875.93 977.50 1002.66 608.95 329.64 612.64 409.43 293.45 222.59 280.70 677.31 605.60 594.37 586.49 464.71 393.10 335.96 302.01 558.95 427.57 344.30 286.16 277.31 241.06 565.27 Smith-supplementn o p q r s t u v w 217 218 554.35 619.26 602.59 416.12 474.37 2670.90 2430.07 1902.55 1826.85 3898.58 495.50 566.80 560.30 361.62 431.80 2599.44 2368.75 1849.51 1784.48 3853.81 613.19 671.71 644.87 470.63 516.94 2742.36 2491.38 1955.59 1869.22 3943.35 18 Smith-supplement219 19 Supplementary Literature Cited 220 221 222 223 224 Anderson, J. F., H. Rahn, and H. D. Prange. 1979. Scaling of Supportive Tissue Mass. Quarterly Review of Biology 54:139-148. Baicich, P. J., and C. J. O. Harrison. 1997. A Guide to the Nests, Eggs, and Nestlings of North American Birds. Academic Press, San Diego, CA. 225 Baker, A. J., S. L. Pereira, and T. A. Paton. 2007. Phylogenetic relationships and divergence 226 times of Charadriiformes genera: multigene evidence for the Cretaceous origin of 14 227 clades of shorebirds. Biology Letters 3:205-209. 228 Bédard, J. 1969. Adaptive radiation in the Alcidae. Ibis 111:189-198. 229 Birkhead, T. R. 1993. Great Auk Islands : a field biologist in the Arctic. Poyser, London. 230 Chapman, W. L. 1965. Appearance of ossification centers and epiphyseal closures as determined 231 by radiographic techniques. Journal of the American Veterinary Medical Association 232 147:138-141. 233 234 Coues, E. 1868. A monograph of the Alcidae. Proceedings of the Academy of Natural Sciences of Philadelphia 20:2-81. 235 Davie, O. 1900. Nests and Eggs of North American Birds. David McKay, Philadelphia. 236 De Santo, T. L., and S. K. Nelson. 1995. Comparative reproductive ecology of the auks(Family 237 Alcidae) with emphasis on the Marbled Murrelet. Pp. 33-47. USDA Forest Service 238 General Technical Report PSW-152. 239 240 241 del Hoyo, J., A. Elliott, and J. Sargatal. 1996. Handbook of the Birds of the World Vol. 3. Hoatzin to Auks. Lynx Edicions, Barcelona. Dunning, J. B. J. 2008. CRC Handbook of Avian Body Masses, 2nd Ed. CRC Press, Boca Raton. Smith-supplement- 20 242 Feilden, H. W. 1872. The birds of the Faroe Islands. The Zoologist 7 (Ser. 2):3277-3294. 243 Field, D. J., C. Lynner, C. Brown, and S. A. F. Darroch. 2013. Skeletal correlates for body mass 244 estimation in modern and fossil flying birds. Plos One 8(11):E82000. 245 doi:10.1371/journal.pone.0082000. 246 Friesen, V., P. Piatt, and A. J. Baker. 1996. Evidence from cytochrome b sequences and 247 allozymes for a new species of alcid: the Long-Billed Murrelet (Brachyramphus perdix). 248 Condor 98:681-690. 249 Fuller, E. 1987. Extinct birds. Cornell University Press, Ithaca NY. 250 Gaston, A. J., and I. L. Jones. 1998. The auks :Alcidae. Oxford University Press, Oxford ; New 251 252 York. Hipfner, J. M., K. B. Gorman, R. A. Vos, and J. B. Joy. 2010. Evolution of embryonic 253 developmental period in the marine bird families Alcidae and Spheniscidae: roles for 254 nutrition and predation? BMC Evolutionary Biology 10:179. 255 http://www.biomedcentral.com/1471-2148/10/179. 256 257 258 259 260 Howard, H. 1970. A review of the extinct avian genus, Mancalla. Los Angles County Museum Contributions in Science 203:1-12. ———. Pliocene avian remains from Baja California. Los Angles County Museum Contributions to Science 217:1-17. Hunter, F. M., I. L. Jones, J. C. Williams, and G. V. Byrd. 2002. Breeding biology of the 261 Whiskered Auklet (Aethia pygmaea) at Buldir Island, Alaska. Auk 119:1036-1051. 262 Kaler, R. S. A., L. A. Kenney, and B. K. Sandercock. 2009. Breeding Ecology of Kittlitz's 263 Murrelets at Agattu Island, Aleutian Islands, Alaska. Waterbirds 32:363-373. Smith-supplement264 265 266 267 21 Konyukhov, N. B., and A. S. Kitaysky. 1995. The asian race of the Marbled Murrelet. Pp. 23-29. USDA Forest Service General Technical Report PSW-152. Ksepka, D. T. 2007. Phylogeny, histology, and functional morphology of fossil penguins (Sphenisciformes). Columbia University. 268 Livezey, B. 1988. Morphometrics of flightlessness in the Alcidae. Auk 105:681-698. 269 Lucas, F. A. 1890. The expedition to Funk Island, with observations upon the history and 270 anatomy of the Great Auk. Report of the United States National Museum for 1887-1888 271 1887-1888:493-529. 272 273 274 Martin, J. W. R., C. A. Walker, R. Bonser, and G. J. Dyke. 2001. A new species of large auk from the Pliocene of Belgium. Oryctos 3:53-60. Nelson, S. K., and T. E. Hamer. 1995. Nest Success and the Effects of Predation on Marbled 275 Murrelets. Pp. 89-97. USDA Forest Service General Technical Report PSW-152. 1995. 276 Nettleship, D. N., and T. Birkhead. 1985. The Atlantic Alcidae: the evolution, distribution, and 277 biology of the auks inhabiting the Atlantic Ocean and adjacent water areas. Academic 278 Press, London and Orlando. 279 Olson, S. L. 1977. A Great Auk, Pingunis [sic], from the Pliocene of North Carolina (Aves, 280 Alcidae). Proceedings of the Biological Society of Washington 90:690-697. 281 Owen, R. 1864. Description of the skeleton of the Great Auk, or Garfowl (Alca inpennis, L.). 282 Prange, H. D., J. F. Anderson, and H. Rahn. 1979. Scaling of Skeletal Mass to Body-Mass in 283 284 285 Birds and Mammals. American Naturalist 113:103-122. Raikow, R. J., L. Bicanovosky, and A. H. Bledsoe. 1988. Forelimb joint mobility and the evolution of wing-propelled diving birds. Auk 105:446-451. Smith-supplement286 22 Ricklefs, R. E., and J. M. Starck. 1998. Embryonic growth and development. Pp. 31-58. In R. E. 287 Ricklefs, and J. M. Starck, eds. Avian Growth and Development. Oxford University Press, 288 New York. 289 290 291 Smith, N. A. 2011b. Taxonomic revision and phylogenetic analysis of the flightless Mancallinae (Aves, Pan-Alcidae). ZooKeys 91:1-116. Smith, N. A., and J. A. Clarke. 2014. Osteological histology of the Pan-Alcidae (Aves, 292 Charadriiformes): correlates of wing-propelled diving and flightlessness. The Anatomical 293 Record 297:188-199. 294 Storer, R. W. 1952. A comparison of variation, behavior and evolution in the sea bird genera 295 Uria and Cepphus. University of California Publications in Zoology 52:121-222. 296 Székely, T., J. D. Reynolds, and J. Figuerola. 2000. Sexual size dimorphism in shorebirds, gulls, 297 and alcids: the influence of sexual and natural selection. Evolution 54:1404-1413.