Chemistry 350 – Inhibitors Problem Set 1. The data below are for an
advertisement

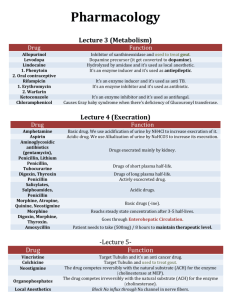
Chemistry 350 – Inhibitors Problem Set 1. The data below are for an enzyme inhibition study. Determine the Km, Vmax for inhibited and uninhibited reactions. Determine the type of inhibition. [S] µM 0.40 0.67 1.00 2.00 V (µmol/min) with 0.0 nM Inhibitor 0.22 0.29 0.32 0.40 V (µmol/min) V (µmol/min) with 25 nM with 50 nM Inhibitor Inhibitor 0.21 0.26 0.30 0.36 0.20 0.24 0.28 0.32 2. The following data were obtained for a competitive inhibition study in which the [I] = 3 uM for each determination of Vo in the presence of inhibitor. Vmax = 200 uM P/min for both data sets. a) Determine Km for the data obtained in the absence of inhibitor. b) Determine Km, app for the data obtained in presence of inhibitor. 3. You have performed a series of experiments determining the Ki values for three competitive inhibitors. Ki (uM) Inhibitor A Ki = 5uM, Inhibitor B Ki = 1uM, Inhibitor C Ki = 0.2uM a) Which inhibitor binds with higher affinity to the free enzyme? b) If the same concentration of inhibitor were used in each experiment, which inhibitor would give the smallest value of Km,app? c) If the value for Km is 1 uM, what is the ratio of Ki/Km for each inhibitor? How is this related to the competing equilibria for binding of the substrate vs. the inhibitor to the enzyme? 4. An enzyme is discovered to be implicated in a disease process. The enzyme is known to have Km = 1.0 mM, and Vmax = 10.0 M/min for its natural substrate. As a therapeutic strategy, a medicinal chemist tries to design a competitive inhibitor for the enzyme. On testing, the inhibitor is shown to be a pure non-competitive inhibitor with Vmax = 1.0 M/min at [I] = 1 mM. Assume [E]total is the same for all the experiments. a)Draw the double reciprocal plot for the enzyme in the presence and absence of the inhibitor. Label which line on the plot corresponds to which experimental trial. b) What is the inhibition constant, KI? c) What would Km have been if the inhibitor had been competitive, assuming the same KI? 5. Phenylketonuria is a genetically determined disease in which the enzyme phenylalanine 4-hydroxylase is absent or has very low activity. The phenylalanine hydroxylase activity of the liver was confined to a single protein of molecular weight of approximately 108,000. It seems to consist of two polypeptide chains, each with a molecular weight of approximately 54,000. By using the double- reciprocal plots, the apparent Km values for phenylalanine were 3.5 x 10-4 M for the full-term infant and 3.8 x 10-4 M for the adult preparations, respectively. For the synthetic cofactor, apparent Km values were 6.8 x 10-5 M and 6.6 x 10-5 M, respectively . The activity of the enzyme was assayed, by using various concentrations of phenylalanine, in the presence and absence of 0.5 mM p-chlorophenylalanine . Iron-chelating and copper-chelating agents inhibited human phenylalanine hydroxylase. Thiol-binding agents inhibited the enzyme but, as with the rat enzyme, phenylalanine both stabilized the human enzyme and offered some protection against these inhibitors. a. The Km value of the enzyme from human fetal liver for phenylalanine was 3.18 x 10-4 M. How does the affinity of the enzyme for the substrate change with development? b. What is the reaction catalyzed by phenylalanine 4-hydroxylase? c. The apparent Km of the enzyme from human fetal liver for the synthetic cofactor was 7.15 x 10-5 M. What changes with development can be inferred from this information? d. What kind of inhibitor would you guess they found p-chlorophenylalanine to be? Why? e. What does the next to the last sentence tell you? f. What does the last sentence tell you?