Example Manuscript for Submission in Pertanika Journal
advertisement

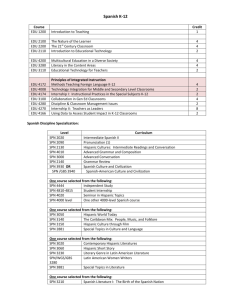
Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) [Subject area] Physics [Figures and Tables] 10 Figures color 1 table black and white List of Figures Fig. 1 The cluster atoms model for Sr58RE6Ti64O192 (X = La, Sm and Er) Fig. 2 Calculated of lattice constant for Sr0.9RE0.1TiO3 (RE = La, Sm and Er) at room temperature Fig. 3 Calculated of lattice constant for Sr0.9X0.1TiO3 (RE = La, Sm and Er) various temperature Fig. 4 Calculated of MSD for Sr0.9X0.1TiO3 (RE = La, Sm and Er) various temperature Fig. 5 Atoms excursion of Sr, Ti, O, La, Sm and Er at temperature 300 K Fig. 6 Atoms excursion of Sr, Ti, O, La, Sm and Er at temperature 1100 K Fig. 7 Pair correlation function (PCF) of (a) OO, TiO and TiTi, (b) SrTi, LaTi, SmTi and ErTi, (c) SrSr, LaSr, SmSr and ErSr, and (d) SrO, LaO, SmO and ErO various distance at temperature 300 K Fig. 8 Pair correlation function (PCF) of (a) OO, TiO and TiTi, (b) SrTi, LaTi, SmTi and ErTi, (c) SrSr, LaSr, SmSr and ErSr, and (d) SrO, LaO, SmO and ErO various distance at temperature 1100 K Fig. 9 Calculated of (a) density and (b) molar volume for Sr0.9RE0.1TiO3 (RE = La, Sm and Er) various temperature Fig. 10 Calculated of (a) potential energy, (b) kinetic energy and (c) entropy for Sr0.9RE0.1TiO3 (RE = La, Sm and Er) various temperature List of table TABLE 1: Interatomic potential function parameter for Sr58RE6Ti64O192 (RE = La, Sm and Er) Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) Meena Rittiruam1, 2, Kunchit Singsoog1, 2 , Hassakorn Wattanasarn1, 2, Athorn Vora-ud1, 2, Suwipong Hemathulin3 and Tosawat Seetawan 1, 2, * 1 Simulation Research Laboratory, Thermoelectrics Research Center, Research and Development Institution, Sakon Nakhon Rajabhat University, 680 Nittayo Road, Mueang District, Sakon Nakhon, 47000, Thailand 2 Program of Physics, Faculty of Science and Technology, Sakon Nakhon Rajabhat University, 680 Nittayo Road, Mueang District, Sakon Nakhon, 47000, Thailand 3 Program of Mechanical and Industrial, Faculty of Industrial Techonology, Sakon Nakhon Rajabhat University, 680 Nittayo Road, Mueang District, Sakon Nakhon, 47000, Thailand E-mail addresses: meena_physics-snru@yahoo.co.th (Meena Rittiruam) kunchitsingsoog@yahoo.com (Kunchit Singsoog) w_hussakorn@hotmail.com (Hassakorn Wattanasarn) a_thorn2008@hotmail.com (Athorn Vora-ud) acumajo@gmail.com (Suwipong Hemathulin) t_seetwan@snru.ac.th (Tosawat Seetawan) *Corresponding Author Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) ABSTRACT Strontium titanate substitute of lanthanide metals Sr0.9RE0.1TiO3 (RE = La, Sm and Er) were simulated by molecular dynamics (MD) method in temperature range 300 K – 1100 K to evaluate the quantum properties viz., lattice constant, density (), molar volume (Vm), mean square displacement (MSD), pair correlation function (PCF), potential energy (U), kinetic energy (Ek) and entropy (S). In MD calculation, we define initial position of 320 atoms (O = 192, Ti = 64, Sr = 58 and X = 6) base on Perovskite structure in cluster atoms site of 4×4×4. The interatomic interaction used the Morsetype potential functions added to the Busing–Ida potential, which composed of Coulomb interactions term, short range repulsion term, van der Waals attraction term and covalent term. We are calculated force by Newton equation of motion, and calculate atom positions and velocities by Verlet’s algorithm. And then evaluate the energy by Ewald’s summation to evaluate quantum properties. It was found that, the lattice constant was decrease form 3.905 Å to 3.8959 Å, 3.8996 Å and 3.8990 Å when substituted of La, Sm and Er, respectively. The PCF of SrO, SrTi and SrSr show that decrease with substituted of La, Sm and Er. Furthermore, the PCF was decreased with increasing temperature. The lattice constant, molar volume, mean square displacement, energy and entropy also increased with increasing temperature, while density decreased and correspond with literature data. The quantum properties of these materials are interesting for feature study thermal properties. Keywords: lattice constant, mean square displacement, pair correlation function, Busing−Ida potential, Morse−type potential, MXDORTO, thermo dynamics, thermoelectric materials Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) INTRODUCTION Strontium titanate (SrTiO3) base on cubic perovskite structure with space group number is 221, space group symbol is Pm 3 m , lattice constant 𝑎 = 3.9050 Å at room temperature (Richet, 1996). Since, SrTiO3 is a n-type oxide thermoelectric (TE) material with a large thermopower > 0.1 mV K1, thermal conductivity of 8 W m1 K1 and ZT of 0.08 at room temperature (Rowe, 2006). To development of TE performance could be several strategies substitutional solid solution formation. The rare earth (RE) was substituted by formula Sr0.9RE0.1TiO3 (RE = Nd, Sm, Gd, Dy, Y, Er, Yb, La) shown that Er have a low thermal conductivity, and Sm high ZT at 1100 K, respectively (Liu, 2013). In addition, the lattice constant was reducing after substitute RE and thus reduces the lattice thermal conductivity (Shang, 2010 and Liu, 2013). Recently, we successes useful molecular dynamics method simulated thermal properties of perovskite structure i.e., SrTiO3 (Seetawan, 2010), Ca0.8M0.2MnO3 (M = Cu; Ag; and Bi) (Seetawan, 2014) and Ca1-XEuXMnO3 (X = 0, 0.05, 0.10, 0.15) (Rittiruam, 2014), which also main focus on thermal conductivity. However, we do not analysis on the thermal properties of crystal such as pair correlation function, mean square displacement, density, molar volume, energy, until the entropy. In this work, we interest in the TE properties of SrTiO3 substitute lanthanides metal. Firstly, we would like study on thermal properties of these material by using computer to simulation before experiment. Which, we choose La, Sm and Er for substitute by formula Sr0.9RE0.1TiO3 to simulation by molecular dynamics method for study thermal properties. Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) COMPUTATIONAL DETAILS The cluster atoms model of Sr0.9X0.1TiO3 were designed by using 320 atoms viz., O = 192, Ti = 64, Sr = 58 and X = 6 atom, respectively, based on perovskite stucture, as show in Fig. 1. In MD calculation, Firstly solving the force for all atomic in the cluster atom by using Newton equation of motion, following eq. (1); Fi mi 2 ri t 2 = U (ri ,..., rN ) ri ; i 1,..., N (1) where mi , ri , t and U are mass of atom i th , position of atom i th , time and potential energy function, respectively. Next, we calculate the positions and velocities in these cluster atoms by using Verlet’s algorithm (Verlet, 1967) base on MXDORTO program (Kawamura, 1994). Time step of 2.0×1015 s was used in calculating velocity. The scaling method was used to control of temperature and pressure. Which, we calculated these cluster atoms in temperature rang 300 K1100 K, pressure in 1 MPa. Then, the energy was evaluated by Ewald’s summation (Wigner, 1932). The cationanion interactions used include of Coulomb interactions, short range repulsion, van der Waals attraction and covalent term. The Morsetype potential function (Morse, 1929) was added in BusingIda potential function ( Ida, 1 9 7 6 ) for interatomic interaction, as given by eq. (2); U ij (rij ) zi z j e2 rij ci c j rij6 ai a j rij f 0 (bi b j )exp bi b j Dij exp 2 ij (rij rij ) 2exp ij (rij rij ) (2) Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) where f0 , zi , z j , rij and rij* , are repulsion betaween atom in vacuum equal 4.186, the effective partial electronic charges on the i th ions, the effective partial electronic charges on the j th ions, inter–atomic distance, bond length of the cation–anion pair in vacuum. a, b and c are the characteristic parameters depending on the ion species. Dij and ij , describes the depth and shape of this potential, respectively. The interatomic potential function parameters for these clusters atomic were carries out, as show in table 1. RESULTS AND DISCUSSIONS The lattice constant of Sr0.9La0.1TiO3, Sr0.9Sm0.1TiO3 and Sr0.9Er0.1TiO3 were carried out by MD calculation with value 3.89590.0023 Å, 3.89960.0022 Å and 3.89900.0021 Å at room temperature, respectively. Comparison of calculated with experiment of the lattice constant shown structure decreased upon substituted La, Sm and Er, which in general of SrTiO3 have lattice constant 3.905 Å at room temperature (Richet, 1996). Another, the lattice constant of these calculated were compared with the calculated result from MXDORTO of SrTiO3 (Seetawan, 2010). It was found that, with increasing temperature the lattice constant of all calculated also increased. The calculated lattice constant of Sr0.9La0.1TiO3, Sr0.9Sm0.1TiO3 and Sr0.9Er0.1TiO3 shows in Fig. 2 and 3. Mean square displacement (MSD) could be calculated from expectation of displacement at initial time to total time, by following equations; MSD [r (t ) r (0)]2 r (t )2 r (0) 2r (0) r (t ) (3) Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) where, r (t ) and r (0) displacement at time t and displacement at initial time, respectively. Figure 4 shows that the atoms of oxygen can large movement when compared with other atoms at room temperature, and then these atoms are tend constant vibration when increasing temperature. On other way, Ti atoms are short movement when compared of other atoms in same temperature, and then it’s tend constant vibration same of oxygen atom. Because of O and Ti were many interaction i.e., TiO, SrO, (La, Sm, Er)O OO, SrTi and (La, Sm, Er)Ti. Moreover, Ti it’s live in centred of the unit cell of SrTiO3, could saw previous in Fig. 1. In addition, the Sr atoms are tending movement quite independently when increasing temperature. After La, Sm and Er were substituted Sr shown increase MSD more than Sr, it’s indicated that of the substitution of lanthanides atom influence to movement and vibration. All of the about movement and vibration were easily descripted by excursion of atoms in unit cell in as show in Fig. 5 and 6. The pair correlation function (PCF) has been descripted by equation (4) (Lowden, 1973); g (r ) 2 N (ri ) (rj r ) (4) i j 1 where and (r ) are density and Dirac delta function. Which the PCF was carried out by MXDORTO. We found that, bonding of TiO, OO and SrTi were indicated explicitly crystallinity, because these bond shown distance of bond by total peak, denote the orderly atomic arrangement. In other peak shown the atoms are neighbours, especially bonding of (Sr, La, Sm, Er)O. By the way, after substitute of La, Sm and Er its shown decrease of total peak of bonding LaO, SmO and ErO, it’s indicated that these substituting affect to decrease crystallinity. In addition, at temperature 1100 K the bonding of ErTi is neighbours by in about rang 1.5 Å2.5Å , Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) and then the bonding of ErO is clearly increase distance from about 1.5 Å at 300 K to about 2.5 Å at 1100 K. Moreover, at above temperature also affect to decrease crystallinity. The PCF of Sr0.9X0.1TiO3 (X = La, Sm and Er) at temperature 300 K and 1100 K shows in Fig. 7 and 8, respectively. The calculated of density of substituted La, Sm and Er had a value 5.288 g cm3, 5.303 g cm3 and 5.40 g cm3 at atm, respectively. Figure 8(a) shows calculated density compared with theoretical density, which these calculate are agreeing with data of Liu (Liu, 2013). We found that, the element of substitute contribution to change the density, it’s dependent on atomic radius and mass. Hence, in case of substitute Er is density more than La and Sm. Moreover, the density and molar volume were compared with temperature, shown density was decreased but molar volume increased with increasing temperature. Hence, the structure had expanded when with increasing temperature. Figure 10 shows the calculated of potential energy, kinetic energy and applied to calculate entropy. The potential was calculated by sum of Coulomb energy and short rang energy, given by; Utotal UCoulomb U short UCoulomb UvdW U srr U Morse (5) where U Coulomb , U vdW , U srr and U Morse are Coulomb potential, van der Waals attraction, short range repulsion and Morse potential energy, respectively. The kinetic energy and entropy can evaluate by equations; N Ek mi vi2 i 3 Nk B H Etotal PV Ek Utotal PV and; PV 2 2 N Ek Nk BT mi vi2 3 3 i (6) (7) (8) Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) N Hence; H U Coulomb U vdW U srr U Morse mi vi2 i 3Nk B 2 N mi vi2 3 i (9) where H , m , v , N and k B are entropy, mass, velocity, total of atom and Boltzmann constant, respectively. It was found, the potential energy of substitute of La, Sm and Er hardly non difference, the kinetic energy also same value in various temperatures. The energy contribution to entropy of system, these work found that entropy little lower potential energy CONCLUSION The MD was used calculate Sr0.9RE0.1TiO3 (RE = La, Sm and Er) to evaluate thermal properties. The thermal properties composed of lattice constant, MSD, PCF, , Vm, U, Ek and S. The lattice constant at room temperature of substitute La, Sm and Er are 3.89590.0023 Å, 3.89960.0022 Å and 3.89900.0021 Å, respectively, were carries out by MD calculation. Which agree with the data experiment of El-Mallah (ElMallah, 2007) and Liu (Liu, 2013). The calculated of MSD, PCF, and Vm had descripted the crystal structure of SrTiO3 decrease after substituted of La, Sm and Er, its according with data of lattice constant. However, the energy and entropy of system do not change. We found the lattice constant, MSD, Vm, U, Ek and S also increased while and PCF decreased at increasing temperature. The quantum properties of these materials are interesting for feature study thermal properties. Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) REFERENCES El-Mallah H. M., & Aziz M. S. (2007). Structural and Conduction Mechanisms Studies in Strontium Lanthanum Titanate Perovskite. Egyptian Journal of Solids, 30(1), 19-29. Kawamura, K., & Hirako, K. (1994). Material design using personal computer. Tokyo: Shokabo. Liu, J., Wang, C. L., Li, Y., Su,W. B., Zhu, Y. H., Li, J. C., & Mei, L. M. (2013). Influence of rare earth doping on thermoelectric properties of SrTiO3 ceramics. Journal of Applied Physics, 114, 223714. Lowden, L., Chandler, D. (1973). Solution of a new integral equation for pair correlation function in molecular liquids. The Journal of Chemical Physics, 59(12), 6587-6595. Morse, P. M. (1929). Diatomic Molecules According to the Wave Mechanics. II. Vibrational Levels. Physics Review, 34, 57-65. Richet, P., & Ligny, P. D. de,. (1996). High-temperature heat capacity and thermal expansion of SrTiO3 and SrZrO3 perovskites. Physical Review B, 53, 3013-3022. Rittiruam, M., Wattanasarn, H., & Seetawan, T. (2014). Thermophysical Properties of Ca1-XEuXMnO3 (X = 0, 0.05, 0.10, 0.15) Simulated by Classical Molecular Dynamics Method. Chiang Mai University Journal of Natural Sciences, 13, 585-593. Rowe, P. D. D. M., (2006). Thermoelectrics Handbook Marco to nano., (p. 35-6). New York: Taylor & Francis Seetawan,T., Wong ud-dee, G., Thanachayanont, C., & Amornkitbumrung, V. (2010). Molecular Dynamics Simulation of Strontium Titanate. Chinese Physics Letters, 27(2), 026501. Seetawan, T. (2014). Theoretical Analysis of the Substitutable Metal on the Thermoelectric Performance of CaMnO3. Integrated Ferroelectrics, 155, 9-14. Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) Shang P., Zhang, B., Li, J., & Ma, N. (2010). Effect of sintering temperature on thermoelectric properties of La-doped SrTiO3 ceramics prepared by solegel process and spark plasma sintering. Solid State Sciences, 12, 1341-1346. Verlet, L. (1967). Experiments on Classical Fluids. I. Thermodynamical Properties of Lennard Jones Molecules. Physics Review, 98-103. Wigner E., (1932). On the Quantum Correction for Thermodynamic Equilibrium. Physics Review, 40, 749-759. Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) Fig. 1 The cluster atoms model for Sr58RE6Ti64O192 (X = La, Sm and Er) Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) 3.904 Lattice constant (Å) 3.902 H.M.El-Mallah (2007) J. Liu et al. (2013) 3.900 3.898 3.896 3.894 T = 300 K, P = 1 MPa 3.892 La Sm Er Sr0.9RE0.1TiO3 (RE = La, Sm and Er) Fig. 2 Calculated of lattice constant for Sr0.9RE0.1TiO3 (RE = La, Sm and Er) at room temperature Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) 3.94 Lattice constant (Å) 3.93 SrTiO3 3.92 3.91 3.90 3.89 La T. Seetawan (2010) 3.88 500 1000 Sm 500 1000 Er 500 1000 Temperature (K) Fig. 3 Calculated of lattice constant for Sr0.9X0.1TiO3 (RE = La, Sm and Er) various temperature Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) 0.5 2 MSD (Å ) 0.4 O 0.3 0.2 Sr 0.1 0.0 La Sm Er Ti 500 1000 500 1000 Temperature (K) Fig. 4 Calculated of MSD for Sr0.9X0.1TiO3 (RE = La, Sm and Er) various temperature Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) La Sr Sm Sr Ti O Ti O 300 K Er Sr Ti O 300 K 300 K Fig. 5 Atoms excursion of Sr, Ti, O, La, Sm and Er at temperature 300 K Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) Sr La Sm Sr Ti Ti O O 1100 K Er Sr Ti O 1100 K 1100 K Fig. 6 Atoms excursion of Sr, Ti, O, La, Sm and Er at temperature 1100 K Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) 2500 800 (a) 2000 600 (b) 500 1500 400 O-O Ti-O Ti-Ti 1000 PCF at T = 300 K Sr-Ti La-Ti Sm-Ti Er-Ti 700 300 200 500 100 0 1.5 2.0 2.5 3.0 3.5 4.0 350 300 250 0 2.5 250 Sr-Sr La-Sr Sm-Sr Er-Sr 3.0 3.5 Sr-O La-O Sm-O Er-O 200 4.0 (d) 150 200 150 100 100 50 0 3.0 50 (c) 3.2 3.4 3.6 3.8 4.0 0 1 2 3 4 Distance (Å) Fig. 7 Pair correlation function (PCF) of (a) OO, TiO and TiTi, (b) SrTi, LaTi, SmTi and ErTi, (c) SrSr, LaSr, SmSr and ErSr, and (d) SrO, LaO, SmO and ErO various distance at temperature 300 K Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) 2500 800 (a) 2000 600 (b) 500 1500 PCF at T = 1100 K Sr-Ti La-Ti Sm-Ti Er-Ti 700 400 O-O Ti-O Ti-Ti 1000 300 200 500 100 0 1.5 2.0 2.5 3.0 3.5 4.0 300 250 0 1 250 350 Sr-Sr La-Sr Sm-Sr Er-Sr (c) 200 200 150 150 100 2 3 Sr-O La-O Sm-O Er-O 4 (d) 100 50 50 0 2.5 3.0 3.5 4.0 0 1 2 3 4 Distance (Å) Fig. 8 Pair correlation function (PCF) of (a) OO, TiO and TiTi, (b) SrTi, LaTi, SmTi and ErTi, (c) SrSr, LaSr, SmSr and ErSr, and (d) SrO, LaO, SmO and ErO various distance at temperature 1100 K (b) 36.4 3 –3 Density (g cm ) 5.35 36.6 –1 (a) 5.40 Molar volume (cm mol ) Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) 5.30 5.25 5.20 5.15 La Sm Er Liu et al. (2013) Liu et al. (2013) 500 36.2 36.0 35.8 La Sm Er 35.6 1000 500 1000 Temperature (K) Fig. 9 Calculated of (a) density and (b) molar volume for Sr0.9RE0.1TiO3 (RE = La, Sm and Er) various temperature Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) –1 70 La Sm Er La Sm Er -6040 -6050 -6060 (a) -6070 500 1000 –1 -5960 60 –1 -6030 Entropy (J mol K ) –1 -6020 -5940 La Sm Er Kinetic energy (J mol K ) –1 –1 Potential energy (J mol K ) -6010 50 40 -5980 -6000 -6020 (c) (b) 30 500 1000 -6040 500 1000 Temperature (K) Fig. 10 Calculated of (a) potential energy, (b) kinetic energy and (c) entropy for Sr0.9RE0.1TiO3 (RE = La, Sm and Er) various temperature Quantum properties of Sr0.9RE0.1TiO3 (RE = La, Sm and Er) TABLE 1: Interatomic potential function parameter for Sr58RE6Ti64O192 (RE = La, Sm and Er) Atom O Ti Sr La O Ti Sr Sm O Ti Sr Er TiO SrO LaO SmO ErO z a (Å) b (Å) For Sr58La6Ti64O192 1.9232 0.16 1.2 1.2 1.055 0.18 1.2 1.198 0.16 1.2 0.6 0.16 For Sr58Sm6Ti64O192 1.926 0.16 1.2 1.2 1.055 0.18 1.2 1.198 0.16 1.2 0.6 0.16 For Sr58Er6Ti64O192 1.9256 0.16 1.2 1.2 1.055 0.18 1.2 1.198 0.16 1.2 0.6 0.16 Atom pair 19 D (10 J) β (Å1) 4.3 3.82 2.41 1.18 2.60 1.18 2.60 1.18 2.60 1.18 c (kJ1/2 Å3 mol1/2) 20 25 10 0 20 25 10 0 20 25 10 0 r* (Å) 2.1923 2.7615 2.7615 2.7615 2.7615