BIO 330 Cell Biology Lecture Outline Spring 2011 Chapter 8

BIO 330 Cell Biology
Lecture Outline
Spring 2011
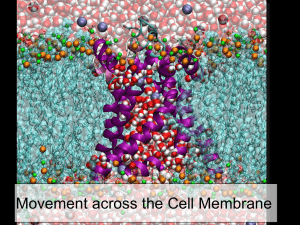
Chapter 8: Membrane Transport
I. Transport Processes
A. Three basic types of processes
Simple diffusion
Facilitated diffusion
Active transport
B. Transport is based on gradients
Concentration gradient
Electrochemical potential (electrochemical “gradient”)
II. Simple Diffusion
A. Definition
The unassisted net movement of a solute from a region where its concentration is higher to a region where its concentration is lower
Hydrophobic core of the membrane prohibits simple diffusion of most solutes
Small nonpolar molecules can move by simple diffusion
E.g., movement of O
2
into and out of erythrocytes
B. Diffusion moves solutes toward equilibrium
Always a spontaneous process
C. Osmosis
D. Characteristics affecting solute diffusion
I.e., requires no energy input
Diffusion proceeds from regions of higher to lower free energy (G)
Definition: Diffusion of water across a selectively permeable membrane
Higher solute concentrations increase entropy and decrease G
Water moves from area of high G to area of low G (typically into cells)
Solute size
MW <100 can diffuse (ETOH, MW 46); MW>100 cannot (glucose, MW 180)
Solute polarity
Partition coefficient – ratio of solubility in organic solvent to solubility in water
High P.C. = high permeability; low P.C. = low permeability
Solute charge
Shell of hydration must be removed prior to diffusion
E. Diffusion rate is directly proportional to concentration gradient
v = P
[S] kinetic properties distinguish simple diffusion from facilitated diffusion non-saturable
III. Facilitated Diffusion
A. Transport proteins
Carrier proteins (transporters; permeases)
BIO 330 Cell Biology
Lecture Outline
Spring 2011
Alternating conformational model
Channel proteins
B. Carrier protein specificity and kinetics
Specificity of carrier proteins
E.g., glucose transporter
Kinetics of carrier function
Saturation kinetics, similar to enzymes
Subject to competitive inhibition
Carrier regulation
External factors can bind and regulate carrier activity
C. Carrier protein solutes
One solute = uniport
Two solutes = coupled transport
Symport (cotransport) vs antiport
E.g., glucose transporter: uniporter
E.g., anion exchange protein: antiporter
D. Channel proteins
Ion channels
Pore lined with hydrophilic amino acid side chains
Highly selective – size and charge
Gated
Voltage-gated
Ligand-gated
Mechanosensitive
Porins
Mitochondria, chloroplasts and bacteria
barrel multipass protein
Aquaporins (AQP)
Involved in water transport
IV. Active Transport
A. Movement up a concentration gradient
Requires input of energy
Thermodynamically unfavorable process is coupled to exergonic process
Allows maintenance of steady state away from equilibrium
Transporters have intrinsic directionality
B. Coupling of active transport to energy source
Direct active transport (primary active transport)
E.g., ATPases
Indirect active transport (secondary active transport)
Movement of 2 solutes: one down its gradient, and one up its gradient
Movement of one down the gradient provides energy for the other
BIO 330 Cell Biology
Lecture Outline
Spring 2011
Gradient for first is established by use of ATP or other energy
Overall decrease in G
C. Four types of ATPases drive direct active transport
P-type ATPases (P = phosphorylation)
Plasma membrane
E.g., Na + /K + ATPase
V-type ATPases (V = vacuole)
Organelles
Proton pumps
F-type ATPases (F = factor)
Bacteria, mitochondria, chloroplasts
Proton transport
2 components, F
0
(transmembrane proton pore) and F
1
(ATP binding site)
Can be run in reverse to make ATP: ATP synthases
ABC-type ATPases (ABC = ATP binding cassette)
Prokaryotic and eukaryotic
Transport variety of solutes: ions, sugars, amino acids, peptides, polysaccharides
E.g., antibiotic resistance transporters
D. Indirect active transport is driven by ion gradients
Sodium ion or proton gradients are used to drive movement of other solutes
Na + /K + ATPase creates Na + gradient; Na + gradient is used to move solutes
Uptake of nutrients; export of Ca2 + , K + wastes
V. Examples of Active Transport
A. Direct transport example
Na + /K + ATPase maintains electrochemical gradients
Conformational change from E1 to E2
Binding of Na + triggers phosphorylation of pump by ATP
Transports 3 Na + out of the cell and 2 K + into cell
Creates electrochemical gradient
B. Indirect transport example
Na + /glucose symporter in intestinal cell
Transports 2 Na+ into cell (down gradient); coupled to transport of glucose into cell from intestinal lumen
VI. Energetics of Transport
A. Neutral solutes: concentration gradient is the only driving force
Think of transport as a chemical equation
G =
G° + RT ln [S] inside
/ [S] outside
K eq
is always 1, so
G° is always zero, so…
G = RT ln [S] inside
/ [S] outside
If [S] inside
is less than [S] outside
then DG is negative = exergonic = spontaneous
BIO 330 Cell Biology
Lecture Outline
Spring 2011
Example – uptake of lactose against a concentration gradient
G = positive, so lactose uptake in endergonic, and requires energy input
Equation as written is for inward transport; flip the fraction for outward transport
B. Charged solutes: electrochemical potential determines transport
Membrane potential, V m
is negative (negative charge on inside of cell)
Favors inward movement of cations & opposes their outward movement
Favors outward movement of anions & opposes their inward movement
To calculate, start with same equation as above, but add a term for electrical potential
G =
G° + RT ln [S] inside
/ [S] outside
+ zFV m
Since V m
is negative, a cation makes zFV m
negative, and makes
G negative
Example – uptake of Cl ions
Concentration gradient drives Cl inward, but electrical potential drives Cl outward