Unit 11 Review Sheet
advertisement
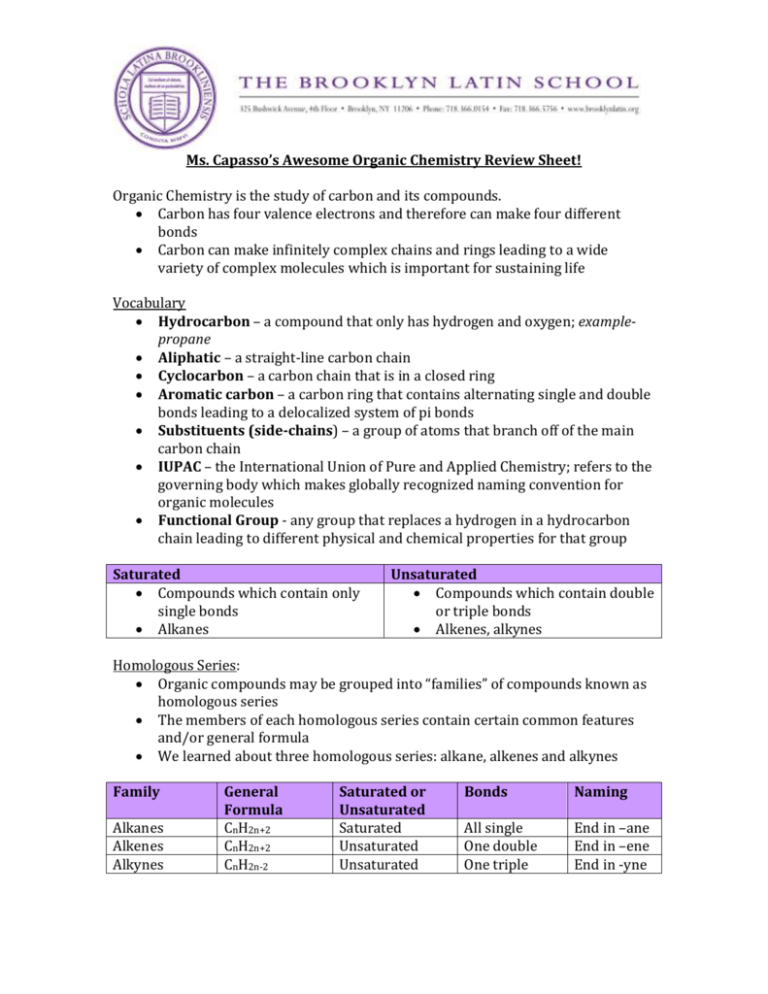
Ms. Capasso’s Awesome Organic Chemistry Review Sheet! Organic Chemistry is the study of carbon and its compounds. Carbon has four valence electrons and therefore can make four different bonds Carbon can make infinitely complex chains and rings leading to a wide variety of complex molecules which is important for sustaining life Vocabulary Hydrocarbon – a compound that only has hydrogen and oxygen; examplepropane Aliphatic – a straight-line carbon chain Cyclocarbon – a carbon chain that is in a closed ring Aromatic carbon – a carbon ring that contains alternating single and double bonds leading to a delocalized system of pi bonds Substituents (side-chains) – a group of atoms that branch off of the main carbon chain IUPAC – the International Union of Pure and Applied Chemistry; refers to the governing body which makes globally recognized naming convention for organic molecules Functional Group - any group that replaces a hydrogen in a hydrocarbon chain leading to different physical and chemical properties for that group Saturated Compounds which contain only single bonds Alkanes Unsaturated Compounds which contain double or triple bonds Alkenes, alkynes Homologous Series: Organic compounds may be grouped into “families” of compounds known as homologous series The members of each homologous series contain certain common features and/or general formula We learned about three homologous series: alkane, alkenes and alkynes Family Alkanes Alkenes Alkynes General Formula CnH2n+2 CnH2n+2 CnH2n-2 Saturated or Unsaturated Saturated Unsaturated Unsaturated Bonds Naming All single One double One triple End in –ane End in –ene End in -yne If the third member of a family is C3H8 than the fifth member is C5H12 Physical Properties – Organic Compounds 1. Mostly non-polar 2. React slower than inorganic compounds 3. Almost all covalent compounds 4. Weak intermolecular forces* (London Dispersion) 5. Low melting point and low boiling point 6. Not soluble in water *The exception being those compounds that contain hydrogen bonds Within a homologous series, as the molecules get bigger, the intermolecular forces (London Dispersion) get stronger and melting point and boiling point get higher! Example: Decane has a much higher boiling point than methane! Formulas for organic compounds: Empirical Shows the simplest whole number ratio of the atoms it contains Example: CH2O Molecular Shows the actual number of atoms of each molecule contained in the compound Example: C6H12O6 Structural Shows how the atoms are bound together Within structural formulas, there are three different types: Full Structural Shows exactly how the atoms are bonded together Condensed Structural Omits showing bonds and instead tries to unambiguously show the structure of the molecule with minimal information Stereochemical Example: CH3CH2CH2CH2CH2CCCH2CH3 is 3-nonyne (alkyne) Attempts to show position of atoms and three dimensional position in space; do not need for Regents Nomenclature – IUPAC Naming IUPAC naming conventions give all scientists a logical way to name organic compounds Steps 1. Identify the longest hydrocarbon chain and give it a prefix (i.e. meth, eth, pro, etc.) 2. Identify the functional groups and give them a name. This can be either some sort of prefix (i.e. cloro, methyl, etc.) or a suffix change (i.e –ol for alcohols) 3. Identify any side chains and give them a name (i.e. methyl, ethyl, etc.) 4. Number your carbons left to right and right to left. Choose the numbering that gives the functional groups, side chains and multiple bonds the lowest numbers possible. Choose only one way! 5. Give each of your functional groups, side chains and multiple bonds a number based on what carbon they are bound to 6. Complete your name by choosing the proper suffix based on the functional group or double/triple bonds Isomers Isomers are compounds that have the same molecular formula but a different structural formula Example: C5H12 Three Isomers Functional Groups Functional groups can be found on Table R Naming conventions can be gathered by looking at Table R; derive their name from the un-substituted carbon chain Functional Group Halide What is it? Alcohol -O-H (hydroxyl group) attached to carbon chain -Br -I -F -Cl attached to a hydrocarbon How is it named? Why is it important? “1-chloro” Used in With a number and refrigerators and a name before the as propellants parent chain Organic solvents and pesticides Changing the -e in Cleaning, fuel and the ending to -ol beverages Ether -O- Aldehyde -C=O H Ketone Organic Acid Ester Common way – Anesthesia (Regents does not go into IUPAC_ name both side chains and then say “ether”. No set rules on which one goes first! Change the final “e” Smell good; vanilla to “al” and cinnamon A carbon with one double bond to an O. Only bound to one other carbon -C=O | E.g. propanal A carbon bound to two other carbons and also double bound to oxygen -COOH E.g. propanone Carbon double bound to an O and single bound to an O-H group -COOCCarbon double bound to an O and single bound to another C and an O Amine -N- Amide Nitrogen bound to carbon with single bonds R-C-N-R’ || O Change the final “e” Great solvents! to “-one” Change the ending to “oic acid” Acids! Used in a lot of biochemical reactions keeping us alive The compound that used to be the acid gets it ending changed to “-oate” and the other named before Smells good – bananas, wintergreen, pineapple, etc. Change final “e” and add “-amine” Important in Biology – B vitamins, hormones, etc. Important for life! Also called a peptide bond because it holds proteins together E.g. methamine Also called a peptide linked. Only when terminal, change final “e” and add “- amide” Alcohols: Can be classified as primary, secondary, or tertiary depending on what carbon the hydroxyl (-OH) group is bound to Can also be classified for how many hydroxyl groups there are in Primary – the carbon –OH is a molecule attached to only attached to one other carbon Secondary – the carbon –OH is attached to attached to two other C’s Tertiary – carbon –OH attached to attached to 3 other C’s Trihydroxyl Alcohol Amino Acids Contain an organic acid group and an amine group Connect together using condensation or dehydration synthesis; resulting bond is an amide Building block of proteins! Organic Reactions Combustion – Burning a hydrocarbon in oxygen until it makes CO2 plus H2O Substitution - Saturated hydrocarbons have all their bonding sites filled with hydrogen. In substitution, one of the hydrogen atoms is replaced with something else (Example: One of the halogens (Group 17) replaces a hydrogen - Br, Cl, or OH) Addition – When two atoms are adding across a double or triple bond Fermentation – Alcohol formed by yeast breaking down sugar; yeast cells secrete an enzyme called zymase which breaks down the six-carbon chain of sugar into CO2 and two alcohol molecules Esterification – Esters form from the reaction of an organic acid and alcohol by the removal of one water molecules Saponification – The hydrolysis of fats by bases to make a soap and a salt; an ester reacts with an inorganic base like NaOH to make soap and a salt Polymerization – Small molecules (monomers) are combined to make larger molecules of repeating units called polymers o Condensation: Polymer forms by the removal of water o Addition: Involves the opening up of double or triple bonds