State Regulations
advertisement

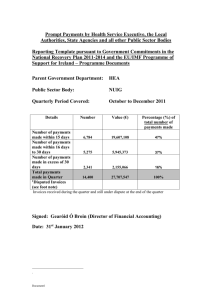
Snapshot: Reporting Requirements/Limitations on Promotional Activities Current as of January 2013 PHrMA. In response to calls for reform from the US Congress and many healthcare organizations, The Pharmaceutical Research and Manufacturers of America (PhRMA) issued a set of recommendations for managing contacts with and payments to healthcare providers, “Code on Interactions with HealthCare Professionals,” in 2009. It is available on the PHrMA Website, http://www.phrma.org/about/principles-guidelines/code-interactions-healthcare-professionals. This document is intended to provide guidance for both promotional and continuing education activities. The Patient Protection and Affordable Care Act (PPACA) was passed by the US Congress in 2010. It contains provisions, specifically section 6002, that mandate applicable manufacturer reporting of payments and other transfers of value to physicians and other healthcare providers. It requires any manufacturer of a covered drug, device, biological, or medical supply that makes a payment to a physician, a physician medical practice, a physician group practice, or a teaching hospital to report annually, in electronic form, specified information on such transactions (any valued over $10) to the Secretary of Health and Human Services. The reporting will go into effect in 2014. Most companies are not limiting reporting to physicians. Drug Company Payment Reporting. Six companies that manufacture oncology drugs or agents have already committed to report payments to doctors, and by implication, to NPs who have prescribing privileges. Novartis and Lilly are reporting all payments to any healthcare providers. These reports are posted on the drug company Websites. Note that company policies vary regarding inclusion of nurses, level of remuneration reported, and more. Most of these reporting commitments are parts of Corporate Integrity Agreements that the companies have with the FDA. Institutional policies vary widely, but many hospitals and other institutions such as medical schools are now requiring payment reporting at a minimum, capping annual totals in other cases, or forbidding all payments for promotional activities entirely (Dana Farber, Emory U.) The American Medical Students Association has prepared a database of institutional policies at teaching hospitals current through 2012. http://www.amsascorecard.org/. The University of Pittsburgh Medical Center (UPMC) has a fairly restrictive policy, “Exemplary” by AMSA standards. Institutions such as Mayo Clinic, Cleveland Clinic, or Dana Farber will not be reflected in the list. All healthcare providers should know and abide by their own employers’ regulations. State Regulations. The chart below reflects the status of state regulations affecting pharmaceutical company payments to healthcare providers as of January 2013. California District of Columbia Maine Massachusetts# Minnesota New Hampshire Nevada Vermont West Virginia Compliance program Spending Limits Gift Restrictions x x $3,500 Gift Reporting Price Disclosure and Reporting x x x Employee and Contractor Reporting x x x x x x x x x $100 x x No use of prescription sales data for marketing to physicians x x $50/modified for meals at programs $50 Advertising Cost Reporting x $0* x x x # Includes both drug and device companies. All payments of $50 or over, for any reason, must be reported. Disclosure becomes public. Promotional payments prohibited, but ad boards may be permitted. In 2012, law was modified to permit “modest” meals in hospital/clinic settings and as part of informational programs. MA providers may now participate in events anywhere in country. * Prescribers may not be paid for any promotional activity; prescribers may attend promotional events only if they pay for their own meals and other expenses For more information, please go to www.onsedge.com/policies, or call 877-588-EDGE (3343, toll-free). Letter from Veteran’s Administration (VA) Outlines Attendance Policy Allowances ONS:Edge has received a letter (2012) from the Department of Veteran Affairs Regional Council 2, Florida District, that asserts: “When there has been a determination that attendance of a VA employee is in the interest of the agency because it will further agency programs and operations, an employee may accept an unsolicited gift of free attendance at all or appropriate parts of a widely attended gathering of mutual interest to a number of parties from the sponsor of the event…Attendance may include waiver of all or part of a conference or other fee or the provision of food, refreshments, entertainment, instruction and material furnished to all attendees as integral part of the event. It does not include travel expenses, lodgings” or other items listed in the letter. VA employees should check with their supervisors, but may attend approved promotional events.