Teachers` Kit - Colby College
advertisement

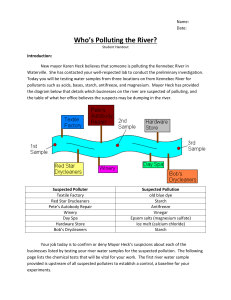
Teacher’s Instructions: Who’s Polluting the River? Recommended grade levels: 7-12, estimated time 1 hour Summary: Students will act as investigative chemists in this lab experiment to determine who is polluting the Kennebec River in Waterville Maine. Using river water samples students will experiment, observe, record, and identify trends to determine the unknown culprit. Common relatively inexpensive materials available from hardware and drug stores are used so that this activity may be conducted on a limited budget. A precipitation reaction, three color change indicators, and a spectrophotometer are used to identify the culprit(s). After collecting data based on their spot plate experiments students are then expected to name the culprit and justify their accusations. Figure 1: Diagram of Kennebec River and suspected polluters with sample collection locations. Teaching Objectives: Communication: students will communicate effectively in the applications of science and technology. Scientific reasoning: students will learn to formulate and justify ideas and to make informed decisions. Inquiry and problem solving: Students will apply inquiry and problemsolving approaches in science and technology by observing and collecting data from their own experimental design Structure of Matter: Students will understand the structure of matter and the changes it undergoes Describe how physical properties of objects change during chemical reactions. Who’s Polluting the River?, Ben Bricker, Colby College, January 2012 Explain how matter changes in both chemical and physical ways Concept Background: pH is a widely used scientific scale used to measure how acidic or basic a solution is. Solutions with a pH less than seven are acidic. Common examples include vinegar and lemon juice. Solutions with a pH greater than seven are basic. These solutions include laundry detergent, ammonia, and bleach. The most common method for calculating the pH of a solution takes the negative log of the concentration (mol/L) of H+ ions. In equation form: pH=-log10([H+])= log10(1/[H+]) For this experiment red cabbage juice is used as a pH indicator. Red cabbage juice contains anthocyanin, a pigment that changes color due to pH. Red cabbage juice is blue in strongly basic pH, bluish-greenish slightly basic pH, purple in neutral pH, and red in acidic pH. Spectrophotometers are also utilized in this experiment. A spectrophotometer consists of two main parts, a spectrometer and a photometer. The spectrometer produces light of specified wavelength. The photometer measures the intensity of the light that reaches it. A space is left between the spectrometer and photometer where a cuvette can be placed. The spectrometer produces light of various wavelengths that passes through the cuvette and sample to the photometer. The photometer measures the amount of light that reaches its detector from the spectrophotometer, and therefore the absorbance of the sample in the cuvette. In this experiment an ocean optics spectrophotometer is used with Vernier data acquisition software. Materials List: Corn Starch Iodine Available at most grocery stores in the baking isle. Available at most grocery stores as an antiseptic in the pharmacy. Acetic Acid White vinegar is available at most grocery stores with the condiments. Magnesium Sulfate Commonly known as Epsom Salts, available at most drug stores. Sold as a topical anesthetic, laxative, or bath additive. Sodium Carbonate Washing soda is a common detergent-booster sold at most grocery stores in the laundry isle. Calcium Chloride Available at most hardware stores. Sold as ice-melt, de-icer, or rock salt (not to be confused with NaCl, which is also sold as rock salt). Red Cabbage Juice Red cabbage from grocery store boiled for approximately 30 minutes and then strained to isolate the reddish-purple juice. Keep refrigerated. Used as a pH indicator. Antifreeze Available at most auto parts store Blue food coloring Available at most grocery stores in the baking isle. Who’s Polluting the River?, Ben Bricker, Colby College, January 2012 Spectrophotometer A scientific instrument for measuring the absorbance of a sample. UV light A simple small black light from a party supply store can be used in place of the more expensive scientific handheld UV light. Spot plates 5mL Pipettes Can be substituted with test tubes, cleaned out children’s paint sets, plastic ice cube trays, or plastic egg boxes. Disposable pipettes are available cheaply in bulk from from any scientific equipment provider. Can also be replaced by straws. Safety Notice: All materials listed are safe to flush down the drain at the end of lab. Vinegar and red cabbage produce strong odors in larger amounts. After preparing solutions do not leave any of the listed materials in unsealed containers. The fumes are not harmful, but the smells will linger. Iodine and red cabbage juice will both stain clothing, and may cause skin discoloration. If a student does accidentally get iodine or red cabbage juice on their skin simply rinse off with tap water. Iodine drops may also leave stains on table/desk tops. Antifreeze is toxic if ingested. Antifreeze alos has a sweet flavor that commonly makes it attractive to small children and pets. If any antifreeze is ingested contact local poison control. Generally, antifreeze must be safely stored and recycled, but due to the relatively small amounts used in this experiment it is safe to flush down the drain. Preparing the Solutions The hardware store is adding calcium carbonate (a common deicer) to the river. 100 mL of water + 22.19g of CaCl2 will yield a solution with a pH of 10 Use the pH indicator red cabbage juice to identify the basic pH. The juice will go from dark purple to light blue-green if the pH is greater than seven. The winery is dumping acetic acid (vinegar) into the river. 100 mL of water + 10mL of white vinegar= pH of 3 Use the pH indicator red cabbage juice to identify the acidic pH. The juice will go from dark purple to dark red. Adding more vinegar will result in a lighter red as the acidity in the solution increases. Who’s Polluting the River?, Ben Bricker, Colby College, January 2012 The day spa is dumping Epsom salts into the river. 100 mL of water + 24.84g of Epsom salts Add 1 Tbs of washing soda to 100mL of water. Add a few drops of this solution to Epsom salt solution to create a white, cloudy precipitate The dry cleaners are adding starch to the river. 10 mL of water + a sprinkling of corn starch Add a drop of iodine to turn the solution dark purple to confirm the presence of starch. The autobody repair business is dumping antifreeze into the river. Antifreeze is colored with a florescent dye that appears to glow under UV light. Expose sample to a black light in a dark space to confirm the presence of antifreeze. 100mL of water + 2mL of antifreeze The textile factory is dumping old blue dye in the river. Use the spectrophotometer to confirm the presence of blue dye. 100 mL of water + 1 drop of blue food coloring The sample spectra will show a significant absorbance peak near 627nm. Figure 2: Example of spectra from a sample containing deionized water with 1 drop of blue food coloring. Note the significant peak close to 627nm. Experimental Setup: Give each group of 3-5 students 15mL test tubes with ~10mL of river water sample. For a simple, quick experiment a single test tube with a river water sample down stream from all suspects can be provided. For a more lengthy procedure, multiple river water samples from different locations may be provided. Teachers can choose which culprit is polluting the river and prepare the river water samples to match (examples on last page). Three test tubes with 2-3mL of iodine, washing soda, and red cabbage juice should also be provided to each group. Preparing test tube racks with the necessary test tubes can be time consuming for the instructor Who’s Polluting the River?, Ben Bricker, Colby College, January 2012 (an additional 20-30 minutes due largely to test tube labeling), but cuts down the amount of time needed for the activity in the classroom while also limiting the potential hazard of students dropping, spilling, or confusing chemicals. Each group should also be provided with at least one spot plate to conduct their investigations and multiple disposable pipettes. Beginning the Experiment: Begin by instructing students that today they are working as investigative chemists attempting to determine who is polluting a specific section of the Kennebec River. As chemists they will be working with chemicals and although everything they will be using is very diluted and relatively harmless, safety precautions must be observed. Everyone must wear safety goggles to protect their eyes and remember to handle their samples carefully. Demonstrating how to use the pipette to transfer a small amount of liquid from a test tube to the spot plate may be necessary if the class is unfamiliar with pipetting. Reminding students that although one pipette may be used to dispense river water into multiple spot plate wells, each reactant must have it’s own pipette. Additionally, if the pipette touches the surface of two samples it cannot be reintroduced to any of the test tubes. For instance, a pipette was used to add red cabbage juice to a spot plate well already containing river water. If the same pipette was then dipped back into the red cabbage juice test tube it may contaminate the stock cabbage juice. Pipettes are meant for one solution only, no mixing. For a one hour experiment students should be given samples from three different sites on the Kennebec. The first sample is upstream of all suspects. This sample is the control. The second sample is downstream of the first drycleaner, the winery, the autobody repair shop, and textile factory. The last sample is downstream of all previous businesses in addition to a day spa, a hardware store, and another drycleaner. Once a group of students believes they have identified the culprit(s) ask them to explain their findings based on experimental evidence. Reactions: Epsom salts and washing soda dissolved in water produce a precipitate, magnesium carbonate. MgSO4(aq) + Na2CO3(aq) ===> MgCO3(s) Corn starch and iodine react to produce a dark blue complex. Beta amylose + I2(aq) ==> I5-1 (a dark blue complex) Red cabbage juice is a natural pH indicator due to the presence of anthocyanin. Red cabbage juice is blue in strongly basic pH, bluish-greenish slightly basic pH, purple in neutral pH, and red in acidic. Experimental Setup Examples: Example 1) Who’s Polluting the River?, Ben Bricker, Colby College, January 2012 The winery, day spa, autobody repair shop, and second drycleaner are polluting the river. 1st river water sample is clean 2nd river water sample contains antifreeze and vinegar 3rd river water sample contains antifreeze, vinegar, starch, and Epsom salts Example 2) The hardware store, first drycleaner, and textile factory are polluting the river. 1st river water sample is clean 2nd river water sample contains blue dye and starch 3rd river water sample contains blue dye, starch, and calcium chloride (making it basic) Example 3) The day spa, textile factory, and second dry cleaner are polluting the river. 1st river water sample is clean 2nd river water sample contains blue dye 3rd river water sample contains blue dye, starch, and Epsom salts. References: Caprette, David R. "Principles of Spectrophotometry." 19 may 2005. Experimental Biosciences Introductory Lab. Januaray 2012 <http://www.ruf.rice.edu/~bioslabs/methods/protein/spectrophotometer. html> Colby College. “Ocean Optics for CH142, 2010.” February 2010. Chemistry 142 General Chemistry Laboratory Materials. January 2012 <http://www.colby.edu/chemistry/CH142L/CH142Lab.html> US Department of Energy. Newton Archives. April 2011. January 2012 <http://www.newton.dep.anl.gov/> Who’s Polluting the River?, Ben Bricker, Colby College, January 2012 Millard, Jullie. "CH151: k-8 Chem Outreach." January 2012. Colby College Department of Chemistry Course Web Pages. January 2012 <http://www.colby.edu/chemistry/CH151_current/CH151_current.html>. Who’s Polluting the River?, Ben Bricker, Colby College, January 2012