MCB_Chap23
advertisement

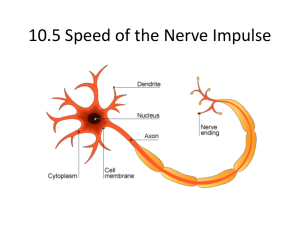
Nerve Cells Nov. 18, 2010 Prof. S. Chung (schung@med.skku.ac.kr) Movie-nervous system, a unique universe: Cellular Microtubule Cytoskeleton in the Adult Mouse Cerebral Cortex. (30 × 25 × 8 m volume). Volume rendering of α-tubulin immunostaining from 40 LRWhite sections, 200 nm each. Dendritic processes can be seen coming out of a cell body, the nucleus of which is devoid of tubulin staining. Lecture Objectives 1. Basic concepts: Hyperpolarization/depolarization, resting membrane potential/ equilibrium (Nernst) potential Threshold, after-hyperpolarization, all-or-none principle of action potential Refractory period (absolute, relative), saltatory conduction of action potential Patch-clamp (voltage-clamp) method 2. Two major reasons for the generation of resting membrane potential. 3. Reversal potential for K+ and Na+ ions. 4. Generation and termination of action potential – voltage-dependent K+ and Na+ ion channels. 5. Origin of threshold of action potential. 6. Refractory period determined the frequency coding of stimulation intensity. 7. Factors determining the speed of action potential propagation. 8. Spatial and temporal summation of action potential Animations for further studies 1. Flow of information through various components of the nervous systems: Reflex Arc http://www.sumanasinc.com/webcontent/animations/content/reflexarcs2.html 2. Mechanism for the generation of resting membrane potential/ action potential http://bcs.whfreeman.com/thelifewire/content/chp44/4401s.swf http://highered.mcgraw-hill.com/sites/0072495855/student_view0/chapter14/animation__the_nerve_impulse.html 3. Voltage-clamp method http://www.sumanasinc.com/webcontent/animations/content/voltage_clamp.html 3. Propagation of action potential – unidirectional vs. bidirectional http://msjensen.cehd.umn.edu/1135/Links/Animations/Flash/0014-swf_action_potenti.swf 4. Factors determining the velocity of action potential: Saltatory conduction: http://www.blackwellpublishing.com/matthews/actionp.html Assignments Q1. Calculate resting membrane potential if K+ permeability is 9 times larger than Na+ permeability? ENa = +62 mV, EK = -80 mV Q2. What are the molecular mechanisms for the selectivity of Na+, K+ channels? Q3. A patient is found to be hypokalemic (low levels of K+) and the physician prescribes K+ supplements. Describe why K+ supplements are important in relation to normal neuronal function. Q4. If a toxin inhibits the action of the Na+/K+ pumps of a neuron, what would it eventually affect the membrane potential? Q5. What will happen in the resting membrane potential if there was an increase or decrease of extracellular K+ concentration? Q6. What will happen in the resting membrane potential if there was an increase or decrease of extracellular Na+ concentration? Q7. Why do most neurons show a sharp rising phase of action potential? Q8. What sets the threshold for action potential? Q9. You are a neurophysiologist recording action potentials from a squid giant axon. Your girlfriend (or boyfriend) is due to visit your lab in 10 minutes. She (or he) has indicated that she (or he) wishes to see “big, big” action potential, overshooting 140 mV above the resting membrane potentials. What would you do to satisfy and impress her (or him)? Explain your idea. Clinical case: 다발성 경화증 (Multiple Sclerosis; MS) 신경섬유를 싸고 있는 수초층이 점점 파괴되어 신경전달이 일시적으로 중단되거나 잘못 전달된다. 특히 시각·감각·팔다리의 움직임과 관련된 신경전달에 이상이 생긴다. 수초층이 떨어져나간 신경조직 때문에 신경전달이 안 되어 영구적인 마비가 올 수도 있다. 전세계에 걸쳐 발생하나 북반구에서 주로 일어나며 대부분 20~40세 사이에 많이 생긴다. 처음에는 팔다 리나 손발을 쓸 때 떨리거나 힘이 없고 눈이 몽롱해지거나 시력이 떨어지며 감각이 이상해지고 걸음걸이가 불안정하며 어지럽고 물체가 2개로 보이고 소변을 못 참는 등의 증상이 일시적으로 나타난다. 이런 초기증 상은 곧 없어지나 몇 달이나 몇 년 뒤에 다시 재발하며 재발할수록 점점 더 심해지고 몇몇 증상은 사라지지 않고 계속된다. 마침내는 운동신경 장애로 완전한 마비를 일으킨다. 증상이 나타난 뒤 평균생존기간은 25년 정도이고 완전히 못 움직이게 된 뒤로도 5년 정도를 산다. 어떤 경우에는 급성으로 진행되어 몇 달만에 급 격히 나빠지기도 한다. 원인이 확실히 밝혀지지 않아서 확실한 치료법은 없고 증상을 완화시키기 위해 부신피질 호르몬제를 쓴 다. 재발된 뒤에는 한동안 쉬며 과로하지 않고 정신적 스트레스를 피하는 것이 좋다. 면역계가 어떤 바이러 스의 침입을 받고 몇 년이 지난 뒤 바이러스 대신 수초층을 잘못 공격하기 때문이라는 연구결과도 있으나 원인이 되는 바이러스를 밝혀내는 데는 실패했다. http://www.livingpixels.net/ms.html Q10. Two persons, each suffering from MS, may experience very different symptoms of the disease. a) Explain. b) How does the destruction of myelin block conductance of the nerve impulse in an affected nerve fiber? c) Currently, what are the treatments for MS? Na+ channel inactivation – “From cartoon to inactivation gate” FIGURE 1. Fast inactivation in A-type K+ channels: the ball-and-chain mechanism. The cartoon represents functional states of a channel with respect to position of inactivation particle and activation gate: C, closed or activatable state; O, open or conducting state; I , inactivated state. FIGURE 2. Localization of the inactivation gate in the primary sequence of cloned A-type K+ channels. A: single-channel openings and ensemble currents of Shaker B (ShB) channels before (control) and after application of trypsin to cytoplasmic side of an inside-out patch. Currents were elicited by a voltage step to 20 mV after a 1-s prepulse to –100 mV; holding potential was –70 mV. Scale bars are as indicated. B: single-channel openings of deletion mutations in the NH2-terminal region of ShB. Amino acid (aa) sequence (given in 1-letter code) is shown at top, with the first 20 aa marked by a box; bars indicate deletions. Filled bars, deletions that disrupted inactivation; open bars, deletions that left inactivation intact. Single-channel openings were elicited by the same pulse protocol as in A. C: restoration of inactivation in ShB 6-46 channels induced by cytoplasmic application of the NH2terminal inactivation peptides derived from ShB (top) and Raw3 (Kv3.4; bottom). Currents were recorded in response to voltage steps to 50 mV (ShB) or to –25, 0, 25, and 50 mV (Raw3) from a holding potential of –110 mV. Peptide concentrations and scale bars are as indicated. D: ball-andchain model after integration of structural data as obtained from site-directed mutagenesis. [Modified from Hoshi et al. (8), Zagotta et al. (15), and Murrell-Lagnado and Aldrich (11).] Refractory Period and Na+ channel inactivation After opening, Na+ channels spontaneously and rapidly enter the inactivation state. At the peak of the action potential, all Na+ channels become inactivated. When Na+ channels are inactivated, they cannot be immediately opened again (Fig. 3). Figure 3. Na+ channel activation, inactivation, and recovery from inactivation. Recovery from inactivation is a time- and voltage-dependent process, and full recovery usually takes about 3–4 ms. Therefore, it takes about 3–4 ms for all Na+ channels to come out of inactivation in order to be ready for activation (opening) again. The period from the initiation of the action potential to immediately after the peak is referred to as the absolute refractory period (ARP) (see Figs. 4, 5). This is the time during which another stimulus given to the neuron (no matter how strong) will not lead to a second action potential. Thus, because Na+ channels are inactivated during this time, additional depolarizing stimuli do not lead to new action potentials. The absolute refractory period takes about 1-2 ms. After this period, Na+ channels begin to recover from inactivation and if strong enough stimuli are given to the neuron, it may respond again by generating action potentials. However, during this time, the stimuli given must be stronger than was originally needed when the neuron was at rest. This situation will continue until all Na+ channels have come out of inactivation. The period during which a stronger than normal stimulus is needed in order to elicit an action potential is referred to as the relative refractory period (RRP). During the relative refractory period, since pK remains above its resting value, continued K+ flow out of the cell would tend to oppose any depolarization caused by opening of Na+ channels that have recovered from inactivation. Figure 4. Absolute and relative refractory periods. Figure 5. Recovery of excitability. In summary, inactivation of Na+ channels is solely responsible for the absolute refractory period. Both Na+ channel inactivation and the greater than resting pK value are responsible for the relative refractory period. The absolute refractory period is responsible for setting the upper limit on the maximum number of action potentials that can be generated during any given time period. In other words, the absolute refractory period determines the maximum frequency of action potentials that can be generated at any point along the axon plasma membrane. This frequency, in turn, has important physiological implications for how the nervous system can respond to high-frequency stimuli, and also for the ability of the nervous system to send high-frequency signals to effector organs when needed. Propagation of action potential in myelinated axon