NMR,X-RAY,IR - E

Nuclear Magnetic Resonance [NMR]
These are of four types:
1.
Proton Magnetic Resonance [PMR]
2.
Carbon Magnetic Resonance [CMR]
3.
Phosphorous Magnetic Resonance [PMR]
4.
Fluoride Magnetic Resonance
Out of these PMR is widely used in determining structure of organic and bioorganic molecules.
PRINCIPLE:
Like electrons move around nucleus, nucleus also revolve around itself and generate magnetic moment which may align with applied magnetic field strength or align against applied magnetic field strength. In order to flip the proton from stable to unstable state it requires certain amount of energy and that energy lies in radio frequency. The equation is
ν = γH0/2∏ : ν is frequency, γ is Gyromagnetic ratio & ∏ is 3.14
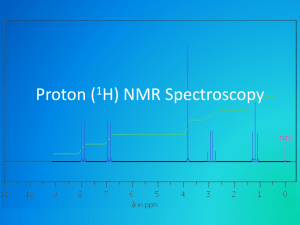
It is more convenient to vary applied magnetic field strength and fix radio frequency. Therefore in NMR spectra the peaks are for a specific proton in organic molecule. In order to determine the chemical shift value [δ], the standard TMS [Tetra Methyl Silane] are used. In tetra methyl silane the electron density around each atom is constant. It is highly soluble in most of the solvents. It is stable. The δ value is arbitrarily fixed as zero. Therefore δ for all protons are been assigned and its value is specific for each proton. For example the primary alcohol proton δ value differs from secondary and tertiary alcohol proton values.
Characteristics features of NMR Spectra are:
1.
Is it upfielded or down fielded.
2.
How many same kind of protons are present in the molecule?
3.
How many different kind of protons present?
4.
The j-j coupling of the peak tells whether the protons are closer to each other.
For example, in ethanol we get three peaks – TMS, Methyl [3 peaks], methylene [ 4 peaks] form right to left in NMR spectra.
Applications:
1.
Denaturation of protein
2.
Exchange of methionine and cyanide in Cytochromes.
3.
Hydrogen bond net work at the distal end is essential for peroxidase catalysis.
References:
1.
Physical chemistry by Puri and Sharma.
2.
Physical Biochemistry by David Friefelder.
3.
Articles that are filed in e-learning files.
FLUORESCENCE SPECTROSCOPY
PRINCIPLE:
Beer – Lambert’s Law: When a beam of monochromatic light is passed through the homogenous solution amount of light emitted by the solute is directly proportional to concentration[c] of the solute and path length of light [l].
FI α cxl
Instrumentation:
Source Slit Monochromator Slit Cuvette
Detector
Recorder
Application:
1.
Determining concentration [Quantitative analysis] of amino acid by fluorescence method.
2.
Qualitative analysis of solutes – Purity analysis
3.
Determination of Kd of E-S complex.
4.
Enzymatic assay
5.
Rate of a reaction.
6.
Identification of cofactor in the solutions.
X- RAY DIFFRACTION STUDIES
Primary structure of protein are predicted by sequencing amino acid present in the protein from amino end to carboxyl end. Secondary structure are predicted by Circular
Dichroism – like % of alpha helix and β-sheets in protein. Tertiary structure are predicted by NMR Spectroscopy and X-ray diffraction studies. Quaternary structure are determined by electron microscopic studies.
The following will be discussed in the class:
1.
Factors affecting crystalisation of proteins.
2.
Methodology – Hanging Drop method- Diagram
3.
Bragg’s law and equation
4.
Conditions for diffraction
5.
Resolution
6.
R-factor
7.
Structure of DNA
8.
Structure of first protein
References:
1.
Protein structure by John Tooze
2.
Protein structure of Myoglobin – a published article
3.
Protein structure of HRP – a published article.