Ch. 13.2 – Saturated Solutions & Solubility
advertisement

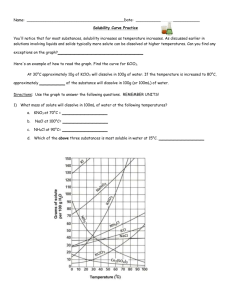
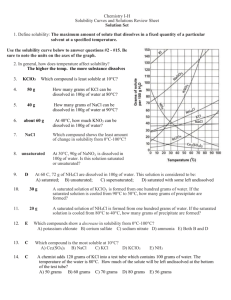
Honors Chemistry Name: _________________________________________ Date:_______________ Mods: ________ Ch. 13.2 – Saturated Solutions & Solubility Dissolution/ Crystallization Equilibrium: o Dissolution – as a soluble solid solute dissolves, the number of solute particles in solution _____________________. o Crystallization – sometimes a dissolved particle will collide with an un-dissolved portion of the solute and will _________________________ to the solid o The dissolution/crystallization possesses are in dynamic ________________________ which means both processes occur at equal rates (the # of particles dissolving is equal to the # of particles recrystallizing) Types of Solutions: (these are _____________________________ dependent) o Unsaturated Solution _______________ than the maximum amount of solute is dissolved (aqueous) at that given temperature The solvent has the capability to dissolve ______________ solute than what it currently has o Saturated Solution The solvent has dissolved the ___________________________ amount of solute that it can at that given temperature No additional solid can be dissolved in a saturated solution therefore, if any more solid is added, it will remain __________________________ at the bottom of the beaker o Supersaturated Solution Solvent holds __________________ than the maximum amount of solute than can be dissolved at that given temperature How is this solution made? …Heat the solvent, add a TON of ____________________, and slowly ______________ the solution so that at the new, cooler temperature your solution is supersaturated! This is an ___________________ solution; crystallization is easily initialed by adding a “seed crystal” or by agitation/movement Saturated Solutions: Concept Questions 1) When a small amount of additional solute is added to an unsaturated solution, what happens to the number of dissolved particles? What does this mean in terms of solution concentration? 2) When a small amount of additional solute is added to a saturated solution, what happens to the number of dissolved particles? What does this mean in terms of solution concentration? 3) The pictures below represent 5 solutions of the same unknown solute “x” at 25ᵒC. Each beaker had a different mass of substance “x” added to 100 mL of water. a) Which beaker(s) contain unsaturated solutions? ______________________________ b) Which beaker(s) contain saturated solutions? ______________________________ c) Are there any beakers for which you cannot tell? ____________________________ If so, why not? d) Predict what would happen to the amount of solid solute at the bottom of beaker E under the following circumstances: More water is added to the beaker: The beaker is heated (assume no evaporation occurs): The beaker sits uncovered for a few days and half of the water evaporates: Table A: Solubility’s of Some Substances in Water at Various Temperatures (each box states the maximum mass, in grams, which can be dissolved in 100 g H2O) 0ᵒC 20ᵒC 50ᵒC 100ᵒC Lithium carbonate Li2CO3 1.5 1.3 1.1 0.70 Potassium chlorate KClO3 4.0 7.4 19.3 56.0 Potassium chloride KCl 27.6 34.0 42.6 57.6 Sodium nitrate NaNO3 74 88.0 114.0 182.0 Lead (II) chloride PbCl2 0.60 0.99 1.70 ---------Cane sugar (sucrose) C12H22O11 179 230.9 260.4 487.0 4) Based on Table A (above), answer the following questions: a) At a temperature of 20ᵒC, 76 g of sodium nitrate were dissolved into 100 g of water. Is this solution considered unsaturated, saturated, or supersaturated? b) 100 g of water are heat to 50ᵒC and then 19.3 grams of potassium chlorate are added. Is this solution considered unsaturated, saturated, or supersaturated? After the 19.3 grams of KClO3 were dissolved in the 100 g of water at 50ᵒC, the solution is allowed to cool down to 20ᵒC. After the cooling, will this solution be considered unsaturated, saturated, or supersaturated? c) Are there any substances listed which decrease in solubility as the temperature of the solution increases? If so, state them below. d) At 0ᵒC, 208 grams of sucrose are added to 100 grams of water and thoroughly stirred. Will all of the sucrose dissolve in the water? Is this sucrose solution considered unsaturated, saturated, or supersaturated? If this sucrose solution were heated to 50ᵒC, would it then be considered unsaturated, saturated, or supersaturated?