Special Import Certificates (SICs) & Special Treatment Certificate
advertisement

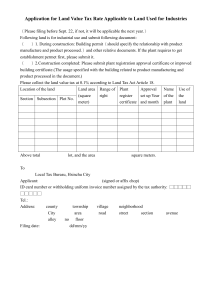
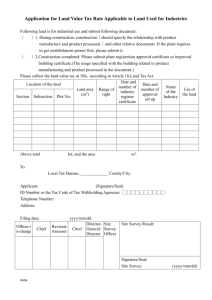
Special Import Certificates (SICs) & Special Treatment Certificate (STCs) Introduction: Scope for Import Certificates Where there is no suitable authorised product in the UK to treat a particular condition and when the health situation so requires, a veterinary surgeon may wish to seek an import certificate to obtain a veterinary medicinal product authorised in another EU Member State or outside the EU. For Special Import Certificates (SICs) it is the responsibility of the veterinary surgeon to justify the use of the product according to the 'prescribing cascade' and keep records to that effect. For Special Treatment Certificates (STCs), no certificate will be granted if there is a suitable UK product, or EU veterinary product available. It is the responsibility of the veterinary surgeon to ascertain this fact. For STCs the VMD must be satisfied that there is a positive benefit: risk assessment that has been performed by the responsible veterinary surgeon, in relation to use of the product in the animal (the justification). The VMD may add specific warnings related to animal or user safety, and environmental safety, but it remains the responsibility of the veterinary surgeon, to perform a full benefit: risk analysis and to ensure safe use and disposal of the product. For these reasons, this may not be a suitable way of obtaining products to treat foodproducing species or for the importation and use of vaccines. Particularly in the case of vaccines, the VMD must have available sufficient information on the quality, manufacture and safety of the product to be certain that no major safety risk will arise. Veterinary specials should also be considered for use under the prescribing cascade. Please see http://www.vmd.defra.gov.uk/mswd/specials.aspx for further information. Q: Do I need a Special Treatment or a Special Import Certificate? A: If the product is to be imported from outside the EU, or is a human product, you will need to apply for a Special Treatment Certificate. If the product has a veterinary authorisation and is being imported from within the EU you will need to apply for a Special Import Certificate. Q: Where can I get an SIC/STC application form from? A: The Special Imports Webpage is itself an online form and we would encourage use of this facility. However if for some reason you are unable to complete the online form you may download SIC and STC forms from the home page of the Special Import Site. When filling out the forms please ensure that all sections are filled out correctly as incomplete application forms will not be validated and this will delay the assessment of your application. Q: What is involved at validation of my application? A: An application is considered valid if it has been entirely completed and (in the case of paper applications) signed by the applicant. When applying to import a product to the UK for the first time, basic data such as Summary of Product Characteristics (in English) and a copy of the product labels will be required prior to the application being considered valid. Q: Other than a completed application form, what additional information should be provided with my SIC/STC application? A: If the application is for a product which the VMD has previously assessed, no further information should be necessary and the application form alone should suffice. However, we VMD/L4/GAT/027/C #444788 may contact you if any detail on the application form is not clear or if we require further clarification on to the justification to import the product. If the application is for a product which the VMD has not previously been assessed, you will initially be asked to supply a Summary of Product Characteristics (SPC) in English and a copy of the labels in their native language for the application to be validated. After this, an assessor may contact you to request further information depending on the nature of the product. In some cases, data on the product will need to be obtained from the manufacturer. Q: If I want to import a live vaccine what extra data do I need to provide: For an application to import a live vaccine under Special Treatment Certificates (STCs) i.e. products not previously imported from outside the EU, the data requirements are: Qualitative and quantitative particulars of the ingredients/materials used in the manufacture of the product. Details on the origin of the strain and information on whether the strain is considered exotic to the UK. If the strain is not considered exotic to the UK, evidence should be provided to confirm this. Information on the genotype of the strain and details on the relationship between this and other know strains. Details on the materials of human and animal origin used to manufacture the product (media, starting materials, etc.), Proof of the stability of the attenuation characteristics of the seeds and data to confirm that there is no risk of reversion to virulence for the vaccine strains. Details of any extraneous agents testing performed on the product. A batch release protocol for a recent batch detailing all the tests conducted on the finished product together with the results of these tests. For an application to import a live vaccine Special Import Certificates (SICs) i.e. products not previously imported from within the EU, the data requirements are: Details on the origin of the strain and information on whether the strain is considered exotic to the UK. If the strain is not considered exotic to the UK, evidence should be provided to confirm this. Information on the genotype of the strain and details on the relationship between this and other know strains. Information on the attenuation characteristics of the products Q: Can we fax our applications to you? A: Unfortunately we can only accept urgent applications by fax. Please do not both fax and post your urgent applications, duplicate applications may be processed and you will be invoiced. If you are unsure that your faxed application has been received, please follow up with a phone call. Q: What is your Fax Number? A: 01932 336618 Q: So what if I need a certificate urgently but the product is not listed as 'urgent'? A: Contact us on 01932 338442 to inform us of the urgency of your application and will attempt to expedite your application. We will email you a copy of the certificate to help you receive the product faster. VMD/L4/GAT/027/C #444788 Q: How much does a certificate cost? A: The costs are as follows: Special Import Certificates handled as hard copy: £15 Special Treatment Certificates handled as hard copy: £30 Repeat Special Treatment Certificates handled as hard copy: £30 There is no fee if the application is made using the special imports page of the VMD website Q: How long will my application take? A: The timescales for dealing with applications are as follows: Normal SIC/STC's: 10 working days from receipt of a valid application. Urgent SIC/STC's: 2 working days (the VMD) have a record of those products which we believe to require a certificate urgently and a fax of the certificate will be provided. 'New' SIC/STC's (for products not imported before): 15 working days from receipt of a valid application. 'New' Urgent SIC/STC's (for urgent products not imported before): 5 working days from receipt of a valid application. Please note these timescales do not include the time taken to request further information from the vet or product manufacturer. Q: Can I get a repeat certificate online? A: You can apply for a repeat Special Treatment Certificate online if you have previously applied by for the same product and animal, and if it states on your certificate that repeats are allowed in the current time frame. Please note that repeat STCs are not permitted for every product, only when the VMD are satisfied that they do not need to reassess the application. When applying online, once the certificate has been generated, it is important to print the certificate straight away otherwise it will automatically be erased. Q: When can I get a repeat certificate? A: Up to one week in advance of the date of repeat specified on your certificate. Repeat certificates are permitted on a case by case basis upon assessment of the original application. E.g. if you are permitted repeats at 6 monthly intervals the date you can download your next certificate will be 6 months from the date on the original certificate and one week prior to this. If there is a supply problem or known delays in receiving stock, contact VMD and we may permit a repeat certificate in advance. VMD/L4/GAT/027/C #444788 Q: I want to apply online but why is my MRCVS number not being recognised? A: You may need to register your RCVS number with VMD if you are new to using the imports scheme. If you're having problems please call Licensing Services on 01932 338442. Q: What should I do if I am using the online site and I have put the wrong quantity in? A: You can use the back button on the webpage to correct a field but do not use the back button on your browser. If you have printed the certificate already please call the VMD for that certificate to be cancelled. Q: What should I do if I have received my certificate in the post but then realise I applied for the wrong amount? A: If any aspect of your certificate is wrong contact the VMD as soon as possible and we will rectify the mistake. In the case that you now require more product than originally thought an assessor may contact you to discuss this. Any change required to your certificate within 2 weeks of issue will be free of charge. Thereafter a new application may have to be made. Q: There is a certificate naming the animal I am treating but not me as the veterinary surgeon, as my client has moved practice. What should I do? A: You should hold a valid certificate naming the animal in question and you as the responsible veterinary surgeon. Contact the VMD for an updated certificate to be issued. Q: I asked for 35 tablets but got a certificate for 50 tablets, why? A: Minimum pack size is taken into account where this information is known to the VMD. Q: Can I put more than one animal on a Special Treatment Certificate? A: Unless it is a herd / flock / pond (Cattle, pigs, goats, sheep, trout, salmon only), you cannot put more than one animal on an STC application, as we need to assess each individual animal. Horses cannot be considered as a herd. Poultry cannot be used as a species type, but one application can cover a flock of chickens or a flock of turkeys. Q: It says on the application form that a justification is needed, what should this include? A: This should include: The clinical details/history of the animal. Reference to any alternative products authorised in the UK (or EU) and a justification as to why these products cannot be used. Previous relevant treatments. The results of any diagnostic tests which have been performed. Please include all of the above details on EACH application. It is not sufficient to write - "see previous applications" and doing so will only delay the determination of your application. Q. Why can’t I get an STC for Founderguard anymore? VMD/L4/GAT/027/C #444788 A: The VMD announced in July 2012 that importation of Virginiamycin (the active ingredient in Founderguard) will be phased out over the next 2 years and will be banned completely from 30 September 2014. From 1 October 2012 you will no longer be able to obtain STCs for Founderguard for horses that have not previously had the medicine prescribed. From 1 October 2014 you will no longer be able to obtain any STCs for Founderguard. Q: Can withdrawal period be a justification for importing a product when a UK Veterinary Medicinal Product (VMP) exists? A: Withdrawal period will only be considered a justification if the UK authorised product is not indicated for the species in question but the proposed imported product is. Q: I want to import a product that will be used in multiple species, can I apply for all species on one application form? A: It is acknowledged that some products e.g Ketamine 1000, is authorised for use in many species, and it may be desired by Veterinary Surgeons for this product to be held in stock for use in multiple species on a case by case basis. To accommodate this, the VMD have changed the online Import Scheme to allow 'mixed' to be selected as the chosen species for Ketamine 1000 applications. Any paper applications for 'mixed' species should also state which species it is expected the product will be used in. Please note the decision to add 'mixed' as a species type for other products will be made on a case by case basis following assessment. Q: If mixed isn't available for the product I want the online import site gives the option of 'other' for species can I just use that instead of mixed? A: No, this option is to be selected only when one specific species is not listed. 'Other' should not be used in place of 'mixed'. Q: Do I need a new certificate if a client transfers to me from another practice with an SIC/STC already? A: Yes. The certificate must be in the name of the responsible veterinarian for that animal. Q: I want to import a product for research purposes only, what do I need to do? A: Research Import Certificate allows a product/substance to be imported for administration under a licence granted in accordance with the Animals (Scientific Procedures) Act 1986. Contact the Home Office to obtain an ASPA and then use the VMD online import site to get a Research Import Certificate. Q: How long is the certificate valid for? A: All our certificates state ' unless suspended or revoked, all SIC and STC certificates will remain valid until the quantity specified has been imported and used, or the date of expiry whichever is the shorter.' Date of expiry is stated on the certificate and is one year from the date the certificate is issued. Q: What should I do if I use a product but it doesn’t work or the animal has an adverse reaction? A: Please submit a SARRS 'yellow form' in the usual way. Q: I've applied for a WDIC but have been asked by VMD for an SIC/STC too, why? VMD/L4/GAT/027/C #444788 A: WDICs will not be issued for new products without evidence of the demand for the product; this should take the form of an SIC/STC application. Q: There is a supply problem with the UK product, can I import an alternative? Manufacturers have been encouraged to inform the VMD in advance of supply problems with UK authorised products. If other UK products are available these must be considered in advance of importing another product. Where no other UK product exists, SIC or STC applications may be submitted. In some situations the manufacturer of the UK products may have already identified an alternative product, details will be on the supply issues page of the VMD website Imports permitted to cover short term out of stock situations will not be available from the online site as the manufacturer of the UK product will need to confirm the continuing out of stock situation. Q: Can I continue to import product after the approval of Marketing Authorisation (MA) in the UK containing the same active ingredient(s) for the same species (until the expiry of the SIC, STC or WDIC)? A: When a UK alternative product becomes available, the imported product is blocked in the SIC database and do not allow any further certificates to be issued. Existing certificates will remain valid but are limited by time or quantity. Q: Can I sell my stock of imported product after the MA is granted in the UK? A: Imported stock held by vets can be used up but no further product could be imported. No SICs/STCs would be issued so no Wholesale Dealer would be able to sell on any further stock where an SIC/STC did not already exist. Q: The product I want to import under WDIC contains a controlled drug, what do I need to do? A: If the product concerned falls within the scope of The Misuse of Drugs Regulations 2001, in addition to complying with VMD requirements, it is also necessary to fulfil Home Office requirements. If you have in place a Home Office licence to supply Schedule 4 Part I drugs, in addition to a Wholesale Dealers Import Certificate from the VMD, you will need to apply to the Home Office for an import licence to bring the controlled drug into the UK for onward supply. The application form for a Home Office import licence can be found here. Q: When and how do I need to send retrospective use records? A: Retrospective use records are only usually permitted for SIC applications. Your certificate will indicate if you have been permitted to import the product on the basis of submitting monthly reports on usage. Monthly records should be sent to the VMD indicating the quantity of product used in which species against each certificate number. There is a proforma for reporting retrospective records on the home page of the SIC online site titled ‘records of use’ please complete this and email it to importcert@vmd.defra.gsi.gov.uk quoting the certificate number to which the records relate. If you do not send records, future applications will not be approved. Q: I want to hold a product in stock for emergency use but it is not a veterinary medicinal product authorised in the EU. Is this allowed? A: In exceptional circumstances an STC will be issued that will enable a product to be held in stock, it will be on the basis that monthly records of use will be supplied. VMD/L4/GAT/027/C #444788 You will not be permitted to hold vast quantities, just enough to treat the immediate need. It is expected that no more than one month’s worth of stock will be held based on your practice usage. For longer term use on a particular animal, an individual STC application naming that animal should be submitted. Q: What file types can be uploaded on to the electronic application form? Word, Excel and pdf files can be uploaded. If you have another file type which contains data relevant to your application please attach this to an email and send to importcert@vmd.defra.gsi.gov.uk. In your message quote the application number which you will be given once your online form has been submitted. Q: If I am applying to import a product that does not have a manufacturing authorisation number what shall I do? Do not leave the field blank, instead enter ‘not authorised’. Q: How long will it take to complete an online application? If you have all the details of the animal and product to hand, the application process should only take a few minutes. Please note though after 30 minutes of inactivity you will be timed out and will have to start again. Q: Why can’t I enter a number in the ‘total number of animals’ box? If this box is fixed at 1, it is because for that product, species and application type a separate application would need to be made per named animal. Q. I am applying for a product which will be used in an animal that will not enter the food chain food but is a producing species. In this case please send an email setting out this information to importcert@vmd.defra.gsi.gov.uk. In your message quote the application number which you will be given once your online form has been submitted. Q. I am a vet who works in a number of practices, can I get certificates with different addresses on? Yes our database will hold details of your RCVS number, you will be able to relate this to a number of practices. Q. I can’t find the product I want in your drop down list This may mean that the product has not previously been imported to the UK and so you will need to complete a ‘new product’ form. If you believe the product has previously been imported it could be that further importation has been blocked. Please call the VMD on 01932 338442 to confirm. VMD/L4/GAT/027/C #444788