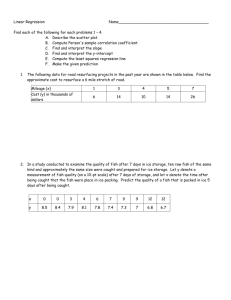
Changes in a tropical marine inshore fish
advertisement

Changes in a tropical marine inshore fish community in a macro-tidal estuary (Darwin Harbour, Australia). This document was prepared for the Department of Land Resource Management. Contributing author Dr Victor Gomelyuk © Copyright of the Northern Territory Government, 2013. Permission to copy is granted provided the source is acknowledged. ISBN: 978-1-74350-061-3 Further information Contact: Dr Victor Gomelyuk Marine Ecosystems, Flora and Fauna Division Dept. of Land Resource Management 564 Vanderlin Drive BERRIMAH NT 0828 Mail to: PO Box 496, PALMERSTON, NT 0831, Australia Ph: +61 8 8995 5024 fax +61 8 8995 5099 Cite report as: Gomelyuk, V. (2013) Changes in a tropical marine inshore fish community in a macro-tidal estuary (Darwin Harbour, Australia). Technical Report for Department of Land Resource Management, Darwin. ii Executive summary Assessing temporal changes and trends in fish communities can assist with management of Darwin Harbour. The diversity and abundance of fish communities were assessed using baited remote underwater system (BRUVS), which uses “video fishing” - recording fish attracted to a camera by standard bait and has been shown to be an effective non-extractive survey method. The approach is useful for long-term environment monitoring because nonimpact nature of visual surveys enables repetitive sampling at reference sites. Fish assemblages were compared between two surveys conducted in 2011 and 2012. Overall, annual differences were relatively small and mainly the result of re-distribution of small school pelagic and demersal species (trevallies, threadfin breams and ponyfishes) rather than an indication of decline in fish abundance and biodiversity in monitored parts of Darwin Harbour. Univariate analyses (ANOVA) of mean fish abundance and the number of species in one video sample appear to be less sensitive and provide very limited insight on the nature of changes in monitored fish assemblages compared to multivariate indices (Permutational multivariate analysis of variance (PERMANOVA), Analysis of Similarity (ANOSIM) and Similarity percentages (SIMPER). Univariate indices therefore have to be used in conjunction with multivariate indices. Further monitoring can add important information of natural temporal variability in fish assemblages of the Harbour. This will increase ability of monitoring to detect and identify changes in fish assemblages caused by adverse environmental and anthropogenic factors. 3 Contents Executive summary ................................................................................................................3 Contents .................................................................................................................................4 1. Introduction .....................................................................................................................9 1.1 Background ..............................................................................................................9 1.2 Obstacles and difficulties in interpreting fish community data in environment health monitoring. ........................................................................................................................10 1.3. 2. Aims .......................................................................................................................11 Material and Methods ...................................................................................................12 2.1 Study design ..........................................................................................................12 2.2 Survey procedure ...................................................................................................16 2.3 Underwater video interrogation...............................................................................16 2.3 Data analysis ..........................................................................................................17 2.3.1 Univariate analyses.............................................................................................17 2.3.2 Multivariate analyses ..........................................................................................17 2.3.2.1 Permutational multivariate analysis of variance (PERMANOVA) .....................18 2.3.2.2 Analysis of similarity (ANOSIM) .......................................................................18 2.3.2.2 Similarity percentages (SIMPER) ....................................................................18 3. Results .............................................................................................................................20 3.1 Descriptive Results.................................................................................................20 3.2 Univariate analyses results .........................................................................................31 North-West site at Channel Island.................................................................................31 Darwin Harbour Entrance site. ......................................................................................31 Rick Mills artificial reef site. ...........................................................................................31 Bottle Washer artificial reef site. ....................................................................................32 3.3 Multivariate analyses results .......................................................................................41 North-West site at Channel Island.................................................................................41 Darwin Harbour Entrance site. ......................................................................................41 Rick Mills artificial reef site. ...........................................................................................41 Bottle Washer artificial reef site. ....................................................................................48 4. DISCUSSION ...............................................................................................................52 5. Acknowledgements.......................................................................................................56 6. References ...................................................................................................................56 7. APPENDIX ...................................................................................................................62 4 5 List of Figures Figure 1. Location of BRUVS monitoring stations in Darwin Harbour. ...................................13 Figure 2. Mean number of species in one 1-hour BRUVS video sample in “outer” and “inner” monitoring stations in 2011 and 2012. Error bars are standard error. Stars between bars indicate significance level: * - p ≤ 0.05; ** - 0.05 > p > 0.01 and *** - p < 0.00 ......................21 Figure 3. Mean number of fish (MaxN) in one 1-hour BRUVS video sample in “outer” and “inner” monitoring stations in 2011 and 2012. .......................................................................22 Figure 4. Different ecological groups of fish in 2011 video records at artificial reefs sites or “outer” monitoring stations in Darwin Harbour. Data labels represent precents of each ecological group of fish to total fish number. .........................................................................29 Figure 5. Different ecological groups of fish in 2012 video records at artificial reefs sites or “outer” monitoring stations in Darwin Harbour.......................................................................29 Figure 6. Different ecological groups of fish in 2011 video records at “inner” monitoring stations in Darwin Harbour. ..................................................................................................30 Figure 7. Different ecological groups of fish in 2012 video records at “inner” monitoring stations in Darwin Harbour. ..................................................................................................30 Figure 8. Fish number, MaxN in BRUVS samples taken at North-West site at Channel Island, "inner" monitoring stations in Darwin Harbour in 2011 - 2012. ...................................33 Figure 9. Fish species number, in BRUVS samples taken at North-West site at Channel Island, "inner" monitoring stations in Darwin Harbour in 2011 - 2012. ...................................34 Figure 10. Fish number, MaxN in BRUVS samples taken at the Entrance site, "inner" monitoring stations in Darwin Harbour in 2011 - 2012. .........................................................35 Figure 11. Fish species number in BRUVS samples taken at the Entrance site, "inner" monitoring stations in Darwin Harbour in 2011 - 2012. .........................................................36 Figure 12. Fish number, MaxN in BRUVS samples taken at Rick Mills site, "outer" monitoring stations in Darwin Harbour in 2011 - 2012. ...........................................................................37 Figure 13. Fish species number in BRUVS samples taken at Rick Mills artificial reef site, "outer" monitoring stations in Darwin Harbour in 2011 - 2012. ..............................................38 Figure 14. Fish number, MaxN in BRUVS samples taken at Bottle Washer artificial reef, "outer" monitoring stations in Darwin Harbour in 2011 - 2012. ..............................................39 Figure 15. Fish species number in BRUVS samples taken at Bottle Washer artificial reef, "outer" monitoring stations in Darwin Harbour in 2011 - 2012. ..............................................40 List of Tables Table 1. The list and description of 12 monitoring stations in Darwin Harbour. .....................14 6 Table 2. Fish species (sorted in accordance to their contribution to total abundance) based on MaxN value during BRUVS survey in 2011-2012 in “inner” monitoring stations” in Darwin Harbour. ...............................................................................................................................23 Table 3. Fish species (sorted in accordance to their contribution to total abundance) based on MaxN value during BRUVS survey in 2011 and 2012 at Bottle Washer and Rick Mills artificial reefs, “outer” monitoring stations. ............................................................................26 Table 4. Permutational MANOVA of Bray-Curtis similarity indices in pooled data of fish assemblages at three monitoring stations at North-West side in Darwin Harbour, 2011-2012 comparison. ..........................................................................................................................42 Table 5. SIMPER comparison of fish assemblages composition at three BRUVS monitoring stations (pooled data) at North-West side of Channel Island in 2011 and 2012. Cut off for low contribution: 90.0%. Average assemblages’ dissimilarity – 81.5%. .......................................43 Table 6. Permutational MANOVA of Bray-Curtis similarity indices in pooled data of fish assemblages at three monitoring stations at the Entrance site in Darwin Harbour, 2011-2012 comparison. ..........................................................................................................................44 Table 7. SIMPER comparison of fish assemblages composition at three BRUVS monitoring stations (pooled data) at the Entrance site in 2011 and 2012. Cut off for low contribution: 90.0%. Average assemblages’ dissimilarity – 82.6%. ...........................................................45 Table 8. Permutational MANOVA of Bray-Curtis similarity indices in pooled data of fish assemblages at three monitoring stations at Rick Mills artificial reef site in Darwin Harbour, 2011 - 2012 comparison. ......................................................................................................46 Table 9. SIMPER comparison of fish assemblages composition at three BRUVS monitoring stations (pooled data) at Rick Mills artificial reef site in 2011 and 2012. Cut off for low contribution: 90.0%. Average assemblages’ dissimilarity –67.5% .........................................47 Table 10 . Permutational MANOVA of Bray-Curtis similarity indices in pooled data of fish assemblages at three monitoring stations at Bottle Washer artificial reef site in Darwin Harbour, 2011-2012 comparison. .........................................................................................49 Table 11. SIMPER comparison of fish assemblages composition at three BRUVS monitoring stations (pooled data) at Bottle Washer artificial reef site in 2011 and 2012. Cut off for low contribution: 90.0%. Average assemblages’ dissimilarity – 78.6%. .......................................50 Table 12. Post hoc power analysis of F tests one-way ANOVA of values of mean fish species number at .............................................................................................................................62 Table 13. Post hoc power analysis of F tests one-way ANOVA of values of mean MaxN at North-West Channel Island site in Darwin Harbour, stations 1-NCHB, 2-NCHB and 4RCKNCH in 2011-2012. ...............................................................................................................62 7 Table 14. Post hoc power analysis of F tests one-way ANOVA of values of mean fish species number at The Entrance site in Darwin Harbour, stations DSAC-B, 8-RCK and 6RCK in 2011-2012. ...............................................................................................................63 Table 15. Post hoc power analysis of F tests one-way ANOVA of values of mean MaxN at .63 Table 16. Post hoc power analysis of F tests one-way ANOVA of values of mean fish species number at Rick Mills artificial reef site in Darwin Harbour, stations RM-1, RM-2 and RM-3 in 2011-2012. ...........................................................................................................................64 Table 17. Post hoc power analysis of F tests one-way ANOVA of values of MaxN at ...........64 Table 18. Post hoc power analysis of F tests one-way ANOVA of values of mean fish species number at Bottle Washer artificial reef site in Darwin Harbour, stations BW-1, BW-2 and BW3 in 2011-2012. ....................................................................................................................65 Table 19. Post hoc power analysis of F tests one-way ANOVA of values of MaxN at Bottle Washer artificial reef site in Darwin Harbour, stations BW-1, BW-2 and BW-3 in 2011-2012. .............................................................................................................................................65 Table 20. ANOVA of number of fish species in one 1-hour BRUVS video sample, pooled data .............................................................................................................................................66 Table 21. ANOVA of number of fish in one 1-hour BRUVS video sample (MaxN), pooled data .............................................................................................................................................66 Table 22. ANOVA of mean fish number (MaxN) in BRUVS samples at three monitoring stations .................................................................................................................................66 Table 23. ANOVA of mean fish species number (FSN) in BRUVS samples at three monitoring ............................................................................................................................66 Table 24. ANOVA of mean fish number (MaxN) in BRUVS samples at three monitoring stations .................................................................................................................................66 Table 25. ANOVA of mean mean fish species number (FSN) in BRUVS samples at three ...67 Table 26. ANOVA of mean fish number (MaxN) in BRUVS samples at three monitoring ......67 Table 27. ANOVA of mean mean fish species number (FSN) in BRUVS samples at three ...67 Table 28. ANOVA of mean fish number (MaxN) in BRUVS samples at three monitoring ......67 Table 29. ANOVA of mean mean fish species number (FSN) in BRUVS samples at three ...68 8 1. 1.1 Introduction Background The direct and indirect coupling between fish communities and human impacts on estuaries reinforces the choice of this taxonomic group as a biological indicator that can assist in the formulation of environmental and ecological quality objectives (Whitfield & Elliott 2002). Many groups of organisms have been proposed and used as indicators of environmental and ecological change (Karr et al. 1986). Fishes have been successfully used as indicators of environmental quality changes in a wide variety of aquatic habitats (Whitfield 1996, SotoGalera et al. 1998). Many studies have looked at fish populations and communities responses on changes in habitat structure (Roberts & Ormond 1987, Tolimieri 1995, Caley & St. John 1996, Friedlander & Parrish 1998, Tolimieri 1998, Holbrook et al. 2000, McClanahan & Arthur 2001, Friedlander et al. 2003). Fishes have numerous advantages fishes as indicator organisms for environmental monitoring programmes: They are usually present in all aquatic systems, with the exception of highly polluted waters; There is extensive life-history and environmental response information available for most species; species are relatively easy to identify; fish communities usually include a range of species that represent a variety of trophic levels; fishes are comparatively long-lived and therefore provide a long-term record of environmental stress; they contain many life forms and functional guilds and thus are likely to cover all components of aquatic ecosystems affected by anthropogenic disturbance; they are both sedentary and mobile and thus will reflect stressors within one area as well as providing groups to give a broader assessment of effects. they have a high public awareness value such that the general public are more likely to relate to information about the condition of the fish community than data on invertebrates or aquatic plants; 9 societal costs of environmental degradation, including cost-benefit analyses, are more readily evaluated because of the economic, aesthetic and conservation values attached to fishes (from Whitfield & Elliott 2002). Within the last two decades fisheries and conservation agencies started to implement more holistic approaches to the assessment and management of resources. The objective has been to move away from single species stock assessment to ecosystem management (ESA 1998; FRCC 1998; NMFS 1999). Using baited remote underwater system (BRUVS), which uses “video fishing” - recording fish attracted to a camera by standard bait, has been shown to be an effective non-extractive survey method to obtain information on a large number of species and individuals in a variety of habitats (Cappo et al. 2003, Watson et al. 2005, Cappo et al. 2007. Dorman, Harvey et al. 2012). Evaluating studies when BRUVS technique was compared with some traditional ichthyological methods (fish traps and trawls) indicated relatively low selectivity of BRUVS (Cappo et al. 2004, Cappo et al. 2007) provide more accurate information on fish assemblages in certain local habitats. BRUVS therefore appears to be an appropriate technique for fish communities/assemblages monitoring. 1.2 Obstacles and difficulties in interpreting fish community data in environment health monitoring. An important problem of any environmental monitoring is the problem of variability and in particular natural variability in time in animals and plants (Morrisey et al. 1992, Underwood 2000). Whitfield & Elliott (2002) listed several key difficulties and problems when fishes are used as indicators of biological integrity: the mobility of fishes on seasonal and diel time scales can lead to sampling bias; fishes may be relatively tolerant to substances chemically harmful to other life forms; fishes can swim away from an anthropogenic disturbance, thus avoiding localized exposure to pollutants or adverse environmental conditions; estuarine environments that have been physically altered by humans may still contain diverse fish assemblages (Whitfield and Elliott 2002). However, even in relatively pristine marine habitats fish community composition and structure can undergo rapid substantial changes due to fish seasonal migration and movements. All mid-water species and many bottom-dwelling species have dial, seasonal movements and 10 simply occasional movements, related to food sources re-distribution (Ommaney 1963). So, changes in species and fish numbers do occur in fish assemblages naturally, without anthropogenic impact and adverse environmental factors and conditions. Therefore, assessment of the range of changes in fish assemblages in “normal” conditions, when sharp changes in anthropogenic impact and influence of adverse environmental factors and conditions are unlikely is essential stage of monitoring. This data is important because it can help to differentiate “natural” variations in fish assemblages structure and changes that may be a result of negative anthropogenic impact (for example, pollution, habitat alteration, overfishing) and adverse environmental factors and conditions (strong storms and tropical cyclones). Knowledge on Northern Territory marine fish assemblages’ composition, structure, seasonal and annual changes are still limited. 1.3. Aims The aims of this study were: a) to assess of natural temporal variability in fish assemblages at each of 12 monitoring stations in Darwin Harbour by comparing samples taken in 2011 and 2012 at the same station; b) to find out by comparing fish assemblages within the same stations what components (species, ecological groups) contribute the most to temporal dissimilarity between assemblages in different years; c) to identify what parameters of fish assemblages are the most perspective for environmental monitoring. 11 2. 2.1 Material and Methods Study design The following criteria used to select stations sites for monitoring included: Fish abundance at the station have to be sufficient (station with poor fish abundance provides less opportunity for detecting changes if fish abundance is in decline). Biodiversity representativeness – station should represent common and wide distributed habitat within the Harbour – rock reefs and coral habitats, areas with deep filter feeders. Water clarity at the station should be similar to overage water clarity in the Harbour and should not be less than 1.5 m to make video analysis and fish identification and count possible. Station should not be under the direct impact of anthropogenic disturbance (e.g. dredging, sewage outfall) in the Harbour. In order to get representation of various habitats of the Harbour four sites were opted for monitoring: one site to south-west of Channel Island with three monitoring stations, representing mid-harbour and bottom part of the Harbour; one site at Harbour entrance with three monitoring stations and two separate sites further in “outer” part of the Harbour close to the Shoal Bay with three monitoring stations at each site. All 12 monitoring stations were selected from 24 sites surveyed in 2011 using stratified random design (Gomelyuk 2012). The shortest distance between two nearest monitoring stations was 250 m. This was done to ensure video replicates were independent (i.e. to exclude possibility of same fish to visit more than one BRUVS device during the survey). Figure 1 represent positions of each station and Table 1 highlights some key environmental characteristics of monitoring stations in Darwin Harbour. 12 Figure 1. Location of BRUVS monitoring stations in Darwin Harbour. 13 Table 1. The list and description of 12 monitoring stations in Darwin Harbour. “ Station Depth Type of bottom habitat Code during name sampling, m “INNER” MONITORING STATIONS North-West Channel Island Location 1 North Channel bank 15.0-15.7 Rocky reef with rich filter feeders 1 (sponges, hydroids, 1-NCHB gorgonians) community and algae 2 4 Rock at North 11-12 Rocky reef with rich filter feeders Channel (sponges, hydroids, 4-RCK- gorgonians) NCHB community and algae 3 North Channel bank 18.0-19.8 Rocky reef with rich filter feeders 2 (sponges, hydroids, 2-NCHB gorgonians) community and algae The Entrance Location 4 DSAC Barge 15-16.5 Rocky reef with rich filter feeders (sponges, hydroids, DSAC-B gorgonians) community, hard corals and algae 5 8 Rock 13.5-17.6 Rocky reef with rich filter feeders (sponges, hydroids, 8-RCK gorgonians) community, hard corals and algae 6 6 Rock 11-12 Rocky reef with rich filter feeders (sponges, hydroids, 6-RCK gorgonians) community, hard corals and algae “OUTER” MONITORING STATIONS Rick Mills Artificial Reef Location 7 8 9 1st station at Rick 12-12.5 Open sandy-muddy bottom ~50 m from 1-RM Mills artificial reef, artificial reef culvert 2nd station at Rick 11-12.5 Open sandy-muddy bottom ~70 m from 2-RM Mills artificial reef artificial reef culvert 3rd station at Rick 12 -12.7 Open sandy-muddy bottom, ~520 m 3-RM Mills artificial reef away from artificial reef culvert Bottle Washer Artificial Reef Location 10 1-st station at Bottle 11-12 Open sandy-muddy bottom ~20 m from 1-BW 14 Washer artificial reef 11 2-nd station at Bottle 11-12 Washer artificial reef 12 3-rd station at Bottle 10-11 Washer artificial reef artificial reef culvert Open sandy-muddy bottom ~570 m 2-BW from artificial reef culvert Open sandy-muddy bottom ~160 m 3-BW away from artificial reef 15 2.2 Survey procedure All surveys were conducted in May–September 2011 and September 2012 during neap tides to avoid high tidal currents and increased water turbidity during spring tides. Surveys were conducted in daytime (between 8:00 hours and 15:00 hours). A Global Positioning System device was used to navigate to each station. The average accuracy of this device is ±5–7 m. Depth was measured using the boat depth sounder. The BRUVS gear used in the survey and the sampling procedure was described in a previous report (Gomelyuk 2013). At each station, one of the BRUVS apparatus was deployed for video survey (Cappo et al. 2003). The BRUVS remained on the sea floor for 60 minutes, and recorded fish attracted to the bait canister. Site name, date, the time when survey was started and finished, the depth and BRUVS device ID number were recorded. Because of relatively large distances between selected monitoring stations and because two BRUVS were never used at the same site, video replicates were independent (e.g. same fish were unable to visit more than one BRUVS during the survey). Six one-hour underwater video samples (i.e. replicates) were taken at each of monitoring station in both years. 2.3 Underwater video interrogation An interrogation of video was done according to Cappo et al. (2003, 2004). All 60 minutes of video were screened and each new fish species arriving in the field of view of the camera was recorded. Only the maximum number of individuals of each species seen together in the field of view at one time (MaxN) was used in analyses to avoid the possibility of fish double counting. According to previous studies (Priede & Merrett 1996; Willis & Babcock 2000 Cappo et al. 2003, 2004; 2007, 20011, Watson et al. 2005), MaxN gives a conservative estimate of fish relative density. Images of the sea bottom were used to identify different types of bottom cover. In Darwin Harbour this can vary from hard coral, rocks, pebbles and gravel to sand, silted sand and silt, depending on the depth and location of survey station. Where possible, fish were identified to species level. Atlas of Living Australia http://biocache.ala.org.au database was used as a main source of information on the previous records of fish species in Darwin Harbour and Northern Territory waters. International Code of Zoological Nomenclature (1999) and the list of standardised Australian fish names http://www.marine.csiro.au/caab/namelist.htm was used in this report. ”Checklist 16 of Darwin Harbour fishes” (Larson 1997) and Atlas of Living Australia http://biocache.ala.org.au/occurrences/search?taxa were used in this study. 2.3 Data analysis 2.3.1 Univariate analyses Univariate statistics and descriptive statistics were completed with SYSTAT 13 software ® (2009 SYSTAT Software Inc., Point Richmond, CA, USA). Analysis of variance (ANOVA) was used to compare temporal differences (i.e., between 2011 and 2012 samples) in the total abundance of fish (MaxN) and species richness (fish species number found in each 1hour sample, FSN). Data were examined for normality and homogeneity of variances using Kolmogorov-Smirnov test (Lilliefors) and Levene's test. In order to decrease possibility of Type I error one fixed factor (factor-different monitoring stations in 2011-2012) was used. Significance level was set at p = 0.05. Pooled data for all “Inner” monitoring stations in 2011 was then compared with 2012 data. Then the data for “outer” stations in 2011 and 2012 was compared. Tukey’s multiple comparison tests (HSD) was used for pairwise comparison between data in 2011 and 2012. A power achieved in F-test and t-test in pairwise comparisons was calculated using G*Power v. 3.1.7 software (Faul et al. 2007, Erdfelder, Lang, & Buchner 2007). Post hoc analysis of achieved power has revealed low (less than 0.8) power values in 60% of all 48 cases of pairwise comparison of FSN in sample and MaxN of single monitoring stations. This result indicated that the probability of incorrectly rejecting the null hypothesis “no difference” between 2011 and 2012 is high. Nevertheless, the power of F-test in one-way ANOVA of sets of pooled data from all three monitoring stations at each site was sufficient in all 4 comparisons (Table 12-19, Appendix). Therefore, further data analyses and discussion was focused on comparison monitoring sites rather than single stations. 2.3.2 Multivariate analyses Descriptive multivariate analyses were calculated with Statistical package PRIMER 6.0 & PERMANOVA + for PRIMER ® (2012 PRIMER-E Ltd., Plymouth, UK, Clarke & Warwick 1994; Anderson, Gorley and Clarke 2008). 17 2.3.2.1 Permutational multivariate analysis of variance (PERMANOVA) PERMANOVA is a program for testing the simultaneous response of one or more variables to one or more factors in an ANOVA experimental design on the basis of any distance measure, using permutation methods. First, the program calculates the distances between each pair of observation units (sampling units) to obtain a distance matrix. It then calculates the test-statistics from this according to the relevant experimental design. (Anderson 2001). One fixed factor (locations=different monitoring stations in 2011-2012) design was used in this analysis. As ANOVA, PERMANOVA comparison of 2011-2012 data was done for sites with pooled data from three monitoring stations at each site. Power of analysis was calculated using Pillai - O'Brien-Shieh algorithm (Erdfelder, Lang, & Buchner 2007). Manova test statistic known as “Pillai’s trace” was calculated during canonical analysis of principal coordinates (CAP), PERMANOVA + Primer package (Anderson et al. 2008). Power values >0.85 were achieved in all 4 comparisons (Appendix, Table 12-19). 2.3.2.2 Analysis of similarity (ANOSIM) The Bray-Curtis similarity matrix was then used to calculate the ANOSIM (analysis of similarity) which depends on the relative order (i.e., ranking) of the similarity coefficients in the Bray-Curtis similarity matrix, rather than their absolute values. The ANOSIM was used to determine whether the fish assemblages differed statistically between years. The ANOSIM test statistic, R, is based on the ratio of the between-group to within-group similarity ranking. Pair wise comparison tests are also calculated which generate an R value and a significance (p) value for each possible pair of comparisons. Generally, the value of R ≥ 0.75 indicates well separated communities, 0.75 > R ≥ 0.5 - overlapping but different communities, 0.5 > R ≥ 0.25 - overlapping but somewhat different and R <0.25 indicates insufficiently different communities (Clarke and Gorley 2001). ANOSIM was done using pooled data of each of three monitoring stations at each site. 2.3.2.2 Similarity percentages (SIMPER) Similarity percentages (SIMPER) were used to examine similarity within the groups (pooled samples collected at three monitoring stations within each site) and to inspect that species contributed to any observed differences between fish assemblages in 2011 and 2012. 18 19 3. Results 3.1 Descriptive Results A total of 3,589 fish from 100 different species was recorded at the 12 monitoring stations in 2011 and 2012 surveys. Fish assemblages parameters differed between sites and areas: mean fish species number in one video sample was almost twice higher at the “outer” monitoring stations with high level of significance (Appendix, Table 20). Mean number of fish (MaxN) in one video sample was almost twice times higher at the “outer” monitoring stations than at “inner” stations on high level of significance (Appendix, Table 21). While no changes were found in mean fish number at “inner” stations, at “outer” stations this index in 2011 was significantly higher comparing to 2012 (Figure 3). This was due to the decrease in numbers of school pelagic trevally, Yellowstripe Scad, Selaroides leptolepis. This fish as well as Fringefin Trevally (Pantolabus radiatus ) and Northwest Threadfin Bream (Pentapodus porosus) dominated video samples and had the highest occurrence at “outer” monitoring stations in 2011 (Tables 2). In 2012, Yellowstripe Scad was recorded only sporadically at some stations (see occurrence percent, Table 2). Both in 2011 and in 2012 four fishes - pelagic school species Yellowstripe Scad and Fringefin Trevally together with demersal (free-swimming near the bottom) Northwest Threadfin Bream and demersal school Whipfin Ponyfish (Equulites leuciscus) were the most abundant in fish assemblages in “outer” Darwin Harbour, comprising more than 50% of all recorded fish (Tables 2, Figures 4). However, in 2012 a notable decrease in number of fish belonging to these ecological groups has been recorded within this site, while the proportion of open bottom areas demersal fish increased (Figure 5, Table 2). 20 Figure 2. Mean number of species in one 1-hour BRUVS video sample in “outer” and “inner” monitoring stations in 2011 and 2012. Error bars are standard error. Stars between bars indicate significance level: * - p ≤ 0.05; ** - 0.05 > p > 0.01 and *** - p < 0.00 21 Figure 3. Mean number of fish (MaxN) in one 1-hour BRUVS video sample in “outer” and “inner” monitoring stations in 2011 and 2012. 22 Table 2. Fish species (sorted in accordance to their contribution to total abundance) based on MaxN value during BRUVS survey in 2011-2012 in “inner” monitoring stations” in Darwin Harbour. Abundance, %1 Species Years 2011 Occurrence, %2 2012 2011 2012 Pantolabus radiatus 20.1 11.7 25.0 36.1 Pentapodus porosus 11.2 16.1 50.0 55.6 Equulites leuciscus 11 17.8 50.0 33.3 Selaroides leptolepis 8.9 0.7 22.2 8.3 Zabidius novemaculeatus 6.1 0.2 16.7 2.8 Lutjanus carponotatus - 4.4 - 33.3 Choerodon cephalotes 3.7 - 25.0 - Caranx sp 3.6 5.5 13.9 30.6 Paramonacanthus choirocephalus 3.1 0.2 27.8 2.8 Choerodon sp. - 3.1 - 13.9 Lutjanus gibbus - 2.9 - 19.4 Nemipterus sp. - 2.9 - 11.1 Upeneus tragula 2.5 - 2.8 - Carcharhinus dussumieri 2.1 1.1 27.8 11.1 Nemipterus nematopus - 2.0 - 25 Choerodon vitta 1.8 2.0 22.2 8.3 Scomberomorus queenslandicus 1.8 - 27.8 - Lutjanus russelli 1.7 - 11.1 - Choerodon schoenleinii 1.7 - 16.7 - Echeneis naucrates 1.5 0.2 16.7 2.8 Lethrinus atkinsoni 1.5 0.9 5.6 11.1 Goby 1.4 0.9 33.3 5.6 Lagocephalus sceleratus 1.4 - 19.4 - Carangoides hedlandensis 0.1 1.3 5.6 8.3 Cephalopholis boenak - 1.1 - 5.6 Carcharhinus dussumieri - 1.1 - 11.1 Sillago sp. 1.2 - 25.0 - Chelmon müelleri 1.2 3.5 5.6 33.3 Flounder 1.2 - 16.7 - Leiognathus sp. - 1.1 - 8.3 23 Chiloscyllium punctatum - 1.1 - 13.9 Gymnocranius elongatus 1 2.0 11.1 19.4 Lutjanus erythropterus 0.9 - 2.8 - Chaetodontoplus duboulayi 0.9 1.5 16.7 13.9 Sphaeramia orbicularis - 0.9 - 8.3 Lethrinus atkinsoni - 0.9 - 11.1 Monacanthus chinensis 0.8 0.2 13.9 2.8 Sphyraena jello 0.7 0.2 8.3 2.8 Choerodon cyanodus 0.7 3.3 8.3 41.7 Hemiscyllium trispeculare - 0.7 - 8.3 Gnathanodon speciosus 0.5 - 5.6 - Nemipterus hexodon 0.5 - 16.7 - Diagramma labiosum 0.5 - 2.8 - Plectropomus laevis - 0.4 - 5.6 Negaprion acutidens 0.4 - 11.1 - Neotrygon kuhlii 0.4 - 11.1 - Diploprion bifasciatum - 0.4 - 5.6 Lagocephalus sp. 0.4 - 11.1 - Sillago sihama - 0.4 - 2.8 Aetobatus narinari - 0.4 - 5.6 Galeocerdo cuvier - 0.4 - 5.6 Carcharhinus melanopterus 0.3 1.3 8.3 13.9 Rhynchobatus djiddensis 0.3 0.2 11.1 2.8 Himantura jenkinsii 0.3 - 2.8 - Ulua aurochs 0.3 - 5.6 - Nemipterus sp. 0.3 - 2.8 - Siganus sp. 0.3 - 2.8 - Nebrius ferrugineus 0.2 - 8.3 - Carcharhinus sp. 0.2 - 8.3 - Sphyrna mocarran 0.2 0.2 5.6 2.8 Cephalopholis boenak 0.2 1.1 2.8 5.6 Cephalopholis sp. - 0.2 - 2.8 Sphyraena obtusata 0.2 - 5.6 - Scorpaenopsis sp. - 0.2 - 2.8 Sphyraena jello - 0.2 - 2.8 Plectorhinchus gibbosus - 0.2 - 2.8 24 Lethrinus nebulosus - 0.2 - 2.8 Sphyraena sp. 0.1 0.2 2.8 2.8 Siganus fuscescens 0.2 - 8.3 - Epinephelus coioides 0.2 2.6 2.8 25.0 Zabidius novemaculeatus - 0.2 - 2.8 Seriolina nigrofasciata 0.1 - 2.8 - Scomberomorus sp. 0.1 - 2.8 - Opistognatus sp. - 0.2 - 2.8 Scarus ghobban - 0.2 - 2.8 Acanthurus grammoptilus - 0.4 - 5.6 1 Species abundance are expressed as a fraction of certain fish species to total number of fish recorded during survey; 2 Species occurrence represents the percent of certain species presence in all samples made in the area. Species contributed to ≥ 90% of total abundance are given in bold. 25 Table 3. Fish species (sorted in accordance to their contribution to total abundance) based on MaxN value during BRUVS survey in 2011 and 2012 at Bottle Washer and Rick Mills artificial reefs, “outer” monitoring stations. Species Abundance, % Years 2011 Occurrence, % 2012 2011 2012 Selaroides leptolepis 24.9 8.9 91.8 22.2 Pantolabus radiatus 16.1 20.1 75.5 25.0 Pentapodus porosus 14.5 11.2 91.8 50.0 Zabidius novemaculeatus 5.7 6.1 36.7 16.7 Equulites leuciscus 5.5 11.0 55.1 50.0 Terapon theraps 3.4 - 24.5 - Scomberomorus sp. 2.4 0.1 59.2 2.8 Caranx sp 1.8 3.6 20.4 13.9 Carangoides sp. 1.6 0.1 38.8 5.6 Nemipterus sp. 1.6 0.3 20.4 2.8 Ambassis sp. 1.4 - 2.0 - Lethrinus sp. 1.3 - 30.6 - Upeneus tragula 1.2 2.5 24.5 2.8 Sillago sp. - 1.2 - 25.0 Chaetodontoplus duboulayi 0.9 0.9 18.4 16.7 Sillago sihama 0.8 - 22.4 - Chelmon müelleri 0.8 1.2 16.3 5.6 Seriolina nigrofasciata 0.7 0.1 20.4 2.8 Lutjanus erythropterus 0.7 0.9 10.2 2.8 Gymnocranius elongatus 0.7 1.0 26.5 11.1 Choerodon cyanodus 0.7 0.7 16.3 8.3 Flounder 0.7 1.2 26.5 16.7 Alectis ciliaris 0.7 - 18.4 - Gnathanodon speciosus 0.6 0.5 12.2 5.6 Choerodon sp. 0.6 - 20.4 - Monacanthus chinensis 0.6 0.8 20.4 13.9 Sphyraena jello 0.6 0.7 16.3 8.3 Carcharhinus dussumieri 0.5 2.1 12.2 27.8 Choerodon cephalotes 0.5 3.7 16.3 25.0 Choerodon vitta 0.5 1.8 10.2 22.2 Paramonacanthus choirocephalus 0.5 3.1 18.4 27.8 Herklotsichthys lippa 0.5 - 8.2 26 Echeneis naucrates 0.5 1.5 12.2 16.7 Lethrinus nebulosus 0.5 - 6.1 - Carcharhinus sp. 0.4 0.2 12.2 8.3 Scolopsis sp. 0.4 - 8.2 - Lethrinus atkinsoni 0.4 1.5 6.1 5.6 Lagocephalus sceleratus 0.4 1.4 8.2 19.4 Negaprion acutidens - 0.4 - 11.1 Neotrygon kuhlii - 0.4 - 11.1 Lagocephalus sp. - 0.4 - 11.1 Carcharhinus dussumieri - 0.3 - 8.3 Lutjanus carponotatus 0.3 - 8.2 - Himantura uarnak 0.3 - 10.2 - Nemipterus hexodon 0.3 0.5 10.2 16.7 Goby 0.3 1.4 10.2 33.3 Ulua aurochs - 0.3 Siganus sp. 0.3 0.3 4.1 2.8 Rhynchobatus djiddensis - 0.3 - 11.1 Himantura jenkinsii - 0.3 - 2.8 Scomberomorus queenslandicus 0.3 1.8 8.2 27.8 Epinephelus lanceolatus 0.2 - 8.2 - Carangoides caeruleopinnatus 0.2 - 2.0 - Cephalopholis boenak 0.2 0.2 4.1 2.8 Siganus fuscescens - 0.2 - 8.3 Diagramma labiosum 0.2 0.5 6.1 2.8 Nebrius ferrugineus - 0.2 - 8.3 Choerodon schoenleinii 0.2 1.7 2.0 16.7 Sphyrna mocarran 0.1 0.2 4.1 5.6 Gymnothorax longinquus 0.1 - 4.1 - Epinephelus coioides 0.1 0.1 4.1 2.8 Lutjanus russelli 0.1 1.7 2.0 11.1 Nemipterus nematopus 0.1 - 2.0 - Paramonacanthus filicauda 0.1 - 2.0 - Chiloscyllium punctatum 0.1 - 2.0 - Carcharhinus sorrah 0.1 - 2.0 - Galeocerdo cuvier 0.1 - 2.0 - Psammoperca waigiensis 0.1 - 2.0 - 5.6 27 Pomadasys maculatus 0.1 - 2.0 - Sphyraena obtusata 0.1 0.2 2.0 5.6 Sphyraena sp. 0.1 0.1 2.0 2.8 Carangoides hedlandensis - 0.1 - 5.6 Anacanthus barbatus 0.1 - 2.0 - Paramonacanthus sp. 0.1 - 2.0 - Lagocephalus lunaris 0.1 - 2.0 - 28 1.3 3.2 Pelagic and demersal school fish species 28.4 Open bottom benthic fish species Reef fish species 67.2 Sharks Figure 4. Different ecological groups of fish in 2011 video records at artificial reefs sites or “outer” monitoring stations in Darwin Harbour. Data labels represent precents of each ecological group of fish to total fish number. 5 3.4 Pelagic and demersal school fish species 41.5 Open bottom benthic fish species Reef fish species 50.1 Sharks Figure 5. Different ecological groups of fish in 2012 video records at artificial reefs sites or “outer” monitoring stations in Darwin Harbour. 29 2.9 26.6 33.3 Pelagic and demersal school fish species Open bottom benthic fish species Reef fish species Sharks 37.2 Figure 6. Different ecological groups of fish in 2011 video records at “inner” monitoring stations in Darwin Harbour. 4.8 Pelagic and demersal school fish species 19 38.9 Open bottom benthic fish species Reef fish species Sharks 37.3 Figure 7. Different ecological groups of fish in 2012 video records at “inner” monitoring stations in Darwin Harbour. 30 The same pelagic and demersal school fish species were also abundant at “inner” Harbour monitoring stations, however reef fishes - Blue Tuskfish (Choerodon cyanodus) Stripey Snapper (Lutjanus carponotatus), Scribbled Angelfish, (Chaetodontoplus duboulayi) and Müller's Coralfish (Chelmon müelleri) and others hard substrate associated species play a substantial role in shaping fish assemblages in “inner” part of the Harbour (Tables 3, Figures 6, 7). Non-school demersal, reef and benthic fish species prevailed in assemblages at “inner” Harbour monitoring stations both 2011 and 2012 (Figures 4, 5). 3.2 Univariate analyses results North-West site at Channel Island. Statistically significant differences were found in 2011 and 2012 comparison in mean fish number in one sample (MaxN) within pooled data from three monitoring stations at this site (p < 0.001, one way ANOVA) (Appendix, Table 22).There were some increase in fish abundance in 2012 (Figure 8). No difference was found between 2011 and 2012 at this site in fish species number, FSN (Figure 9, Appendix, Table 23). Darwin Harbour Entrance site. ANOVA found significant difference in mean fish abundance (MaxN) comparison of data for 2011-2012 (Table 24, Appendix). However, this difference reflects variation in MaxN between the different stations at the site. No statistically significant differences were found between 2011 and 2012 (Figure 10). Similarly, while mean fish species number varied between different stations within the site, however no statistically significant differences were found in 2011-2012 comparison (Figure 11, Appendix, Table 25). Rick Mills artificial reef site. Some increase in in MaxN was found at this site in 2011-2012 comparison, differences statistically significant (p < 0.018, one way ANOVA) (Figure 12, Appendix, Table 26). Contrary to the mean fish abundance, some decrease in mean number of fish species was recorded at this site site in 2012 (p < 0.001, one way ANOVA) (Figure 13, Appendix, Table 27). 31 Bottle Washer artificial reef site. A notable decrease in MaxN value was found in 2012 in interannual ANOVA comparison, differences significant, p = 0.001 (Figure 14, Appendix, Table 28). Although ANOVA also found significant differences in mean fish species number comparison of samples taken at this site (p = 0.018, Apendix, Table 29), this difference reflects spatial variability between monitoring sites rather than interannual changes (Figure 16). 32 Figure 8. Fish number, MaxN in BRUVS samples taken at North-West site at Channel Island, "inner" monitoring stations in Darwin Harbour in 2011 - 2012. 33 Figure 9. Fish species number, in BRUVS samples taken at North-West site at Channel Island, "inner" monitoring stations in Darwin Harbour in 2011 - 2012. 34 Figure 10. Fish number, MaxN in BRUVS samples taken at the Entrance site, "inner" monitoring stations in Darwin Harbour in 2011 - 2012. 35 Figure 11. Fish species number in BRUVS samples taken at the Entrance site, "inner" monitoring stations in Darwin Harbour in 2011 - 2012. 36 Figure 12. Fish number, MaxN in BRUVS samples taken at Rick Mills site, "outer" monitoring stations in Darwin Harbour in 2011 - 2012. 37 Figure 13. Fish species number in BRUVS samples taken at Rick Mills artificial reef site, "outer" monitoring stations in Darwin Harbour in 2011 - 2012. 38 Figure 14. Fish number, MaxN in BRUVS samples taken at Bottle Washer artificial reef, "outer" monitoring stations in Darwin Harbour in 2011 - 2012. 39 Figure 15. Fish species number in BRUVS samples taken at Bottle Washer artificial reef, "outer" monitoring stations in Darwin Harbour in 2011 - 2012. 40 3.3 Multivariate analyses results North-West site at Channel Island. Significant difference in fish assemblages compositions based on Bray-Curtis similarity was found during Permutation MANOVA comparison of samplings done in 2011 and in 2012, (Table 4). However, Global R calculated during ANOSIM was 0.202 at significance level of p=0.01. This indicates insufficient differences in community composition in 2011 and 2012. SIMPER analysis showed that major contributors (60.8%) to changes in fish assemblages compositions at this site in 2011-2012 were six fish species – bottom school ponyfish Leiognathus sp. and Northwest Threadfin Bream Pentapodus porosus, pelagic trevally Caranx sp. and Fringefin Trevally Pantolabus radiates. Only two reef fish - Stripey Snapper Lutjanus carponatus and Blue Tuskfish Choerodon cyanodus were in this contributors group (Table 5). Darwin Harbour Entrance site. Permutation MANOVA comparison of Bray-Curtis similarity index of fish assemblages at at the Entrance site in 2011 and 2012 found difference of high significance level (Table 6). Yet again ANOSIM Global R value was relatively low – 0.172, p = 0.07 denoting minor differences in community composition between 2011 and 2012. According to SIMPER analysis, 6 species contributed to ~50% of differences in community composition and structure between 2011 and 2012. These species were school bottom Northwest Threadfin Bream Pentapodus porosus and ponyfish Leiognathus sp., pelagic Fringefin Trevally Pantolabus radiatus and reef species Restripe Tuskfish Choerodon vitta, Stripey Snapper Lutjanus carponatus and Blue Tuskfish Choerodon cyanodus (Table 7). Rick Mills artificial reef site. Permutation MANOVA indicated that the difference in Bray-Curtis similarity index between BRUVS samples collected at three monitoring stations at this site in 2011 and 2012 was statistically highly significant (Table 8). 41 Table 4. Permutational MANOVA of Bray-Curtis similarity indices in pooled data of fish assemblages at three monitoring stations at North-West side in Darwin Harbour, 2011-2012 comparison. Source df Stations*Year 1 34 Res Total 35 SS 11619 MS Pseudo-F 11619 3.7465 1.0544∙E5 3101.2 P(perm) Unique permutations, N 0.004 999 1.1706∙E5 42 Table 5. SIMPER comparison of fish assemblages composition at three BRUVS monitoring stations (pooled data) at North-West side of Channel Island in 2011 and 2012. Cut off for low contribution: 90.0%. Average assemblages’ dissimilarity – 81.5%. Years 2012 Abundance, N 3.39 2011 Abundance, N 0.33 Caranx sp. 1.39 1.28 9.41 11.49 Pantolabus radiatus 2.22 0.06 8.70 10.63 Pentapodus porosus 1.28 0.78 7.13 8.72 Lutjanus carponotatus 0.78 0.89 6.20 7.57 Choerodon cyanodus 0.44 1.39 5.95 7.27 Lutjanus gibbus 0.72 0.11 4.92 6.01 Chelmon müelleri 0.39 0.94 4.90 5.98 Epinephelus coioides 0.44 0.72 4.80 5.87 Chaetodontoplus duboulayi 0.06 0.89 4.64 5.67 Sphaeramia orbicularis 0.22 0.17 1.75 2.14 Chiloscyllium punctatum 0.22 0.11 1.70 2.08 Plectorhinchus gibbosus 0.06 0.22 1.18 1.44 Species Leiognathus sp. Dissimilarity, % 12.44 Contribution, % 15.20 43 Table 6. Permutational MANOVA of Bray-Curtis similarity indices in pooled data of fish assemblages at three monitoring stations at the Entrance site in Darwin Harbour, 2011-2012 comparison. Source df Stations*Year 1 SS 11619 MS Pseudo-F 11619 3.7465 Res 34 1.0544∙E5 3101.2 Total 35 1.1706∙E5 P(perm) Unique permutations, N 0.004 999 44 Table 7. SIMPER comparison of fish assemblages composition at three BRUVS monitoring stations (pooled data) at the Entrance site in 2011 and 2012. Cut off for low contribution: 90.0%. Average assemblages’ dissimilarity – 82.6%. Years 2012 2011 Species Pentapodus porosus Abundance, N 3.39 Abundance, N 1.56 Leiognathus sp. 1.33 1.83 7.98 9.65 Pantolabus radiatus 0.72 2.67 7.01 8.49 Choerodon vitta 1.22 0.17 5.27 6.38 Lutjanus carponotatus 0.39 0.89 4.09 4.94 Choerodon cyanodus 0.78 0.78 3.99 4.83 Nemipterus sp. 0.17 0.89 3.71 4.48 Chaetodontoplus duboulayi 0.5 0.72 3.52 4.26 Chelmon müelleri 0.28 0.5 2.67 3.23 Choerodon cephalotes 0.28 0.44 2.34 2.83 0 0.5 2.08 2.52 Carcharhinus dussumieri 0.28 0.5 2.04 2.47 Acanthurus grammoptilus 0.33 0.17 1.8 2.18 Epinephelus coioides 0.28 0.33 1.74 2.1 Nemipterus nematopus 0.39 0.11 1.74 2.1 0 0.28 1.61 1.94 Lethrinus atkinsoni 0.11 0.33 1.48 1.79 Cephalopholis boenak 0.28 0.17 1.44 1.75 Plectropomus maculatus 0.11 0.22 1.25 1.51 Pomacanthus sexstriatus 0 0.28 1.1 1.33 0.33 0 1.07 1.3 0 0.28 1.04 1.26 Hemiscyllium trispeculare 0.17 0 0.92 1.12 Gymnocranius elongatus 0.17 0.06 0.71 0.86 Carangoides caeruleopinnatus 0 0.17 0.68 0.83 Plectorhinchus gibbosus 0 0.17 0.66 0.8 Lethrinus laticaudis Choerodon sp. Carangoides hedlandensis Lutjanus vitta Dissimilarity, % 12.6 Contribution, % 15.24 45 Table 8. Permutational MANOVA of Bray-Curtis similarity indices in pooled data of fish assemblages at three monitoring stations at Rick Mills artificial reef site in Darwin Harbour, 2011 - 2012 comparison. Source df Stations*Year 1 SS 9266.1 MS Pseudo-F 9266.1 4.7329 Res 34 66566 1957.8 Total 35 75832 P(perm) Unique permutations, N 0.001 998 46 Table 9. SIMPER comparison of fish assemblages composition at three BRUVS monitoring stations (pooled data) at Rick Mills artificial reef site in 2011 and 2012. Cut off for low contribution: 90.0%. Average assemblages’ dissimilarity –67.5% Years 2012 2011 Species Pantolabus radiatus Abundance, N 9.83 Abundance ,N 11.83 Dissimilarity, % 15.21 Contribution, % 22.51 Selaroides leptolepis 3.5 10.33 9.73 14.41 Pentapodus porosus 2.33 6.89 7.1 10.51 Zabidius novemaculeatus 2 2.33 4.3 6.36 Caranx sp. 1.5 1.39 3.14 4.65 Upeneus tragula 1.33 0.83 2.42 3.58 Scomberomorus queenslandicus 0.39 1.44 1.78 2.64 Choerodon cephalotes 1.22 0.56 1.34 1.98 Lutjanus erythropterus 0.5 0.61 1.28 1.9 Lethrinus atkinsoni 0.72 0.28 1.26 1.86 Leiognathus sp. 0 0.67 1.23 1.81 Chelmon müelleri 0.5 0.5 1.06 1.58 Carangoides sp. 0 0.67 1 1.49 Lethrinus sp. 0 0.72 0.89 1.32 Gymnocranius elongatus 0.39 0.39 0.87 1.28 Scolopsis sp. 0 0.5 0.84 1.24 Chaetodontoplus duboulayi 0.17 0.44 0.84 1.24 Carcharhinus dussumieri 0.5 0.17 0.83 1.23 Sphyraena jello 0.28 0.5 0.77 1.14 Lutjanus russelli 0.61 0 0.73 1.08 Flounder 0.28 0.28 0.68 1.01 Paramonacanthus choirocephalus 0.33 0.28 0.67 0.99 Choerodon vitta 0.44 0.11 0.64 0.95 Nemipterus sp. 0.17 0.28 0.62 0.91 Echeneis naucrates 0.22 0.28 0.59 0.88 Diagramma labiosum 0.28 0.17 0.52 0.77 Siganus fuscescens 0.17 0.28 0.5 0.74 47 The Global R value in ANOSIM was low – 0.223, p = 0.01, suggesting only a slight difference in comparing fish assemblages. SIMPER analysis results showed that changes in abundance of just five fish species of all 29 recorded at Rick Mills artificial site contributed to 58.5% of dissimilarity between assemblages of 2011 and 2012. These fish were pelagic school Fringefin Trevally Pantolabus radiates, Yellowstripe Scad Selaroides leptolepis, trevally Caranx sp. and Shortfin Batfish Zabidius novemaculeatus, Bottom Northern Treadfin Bream was also in this the most contributing group (Table 9). Bottle Washer artificial reef site. According to Permutation MANOVA, were was a significant difference in Bray-Curtis similarity index between samples collected at Bottle Washer artificial reef site in 2011 and 2012 (Table 10). ANOSIM Global R calculated for this site was 0.375, p = 0.01. Such value of sample statistic indicates “overlapping but somewhat different communities” (Clarke and Gorley 2001) at this site in 2011 and 2012. SIMPER analysis showed that the leading role in contribution to 52.6% of dissimilarity between fish assemblages 2011 and 2012 was played by only 5 species - pelagic school species - Yellowstripe Scad Selaroides leptolepis, Fringefin Trevally Pantolabus radiates and Shortfin Batfish Zabidius novemaculeatus, followed by bottom ponyfish Leiognathus sp. and Northern Treadfin Bream Pentapodus porosus (Table 11). 48 Table 10 . Permutational MANOVA of Bray-Curtis similarity indices in pooled data of fish assemblages at three monitoring stations at Bottle Washer artificial reef site in Darwin Harbour, 2011-2012 comparison. Source df Stations*Year 1 SS 15596 MS Pseudo-F 15596 6.4447 Res 34 82276 2419.9 Total 35 97872 P(perm) Unique permutations, N 0.001 997 49 Table 11. SIMPER comparison of fish assemblages composition at three BRUVS monitoring stations (pooled data) at Bottle Washer artificial reef site in 2011 and 2012. Cut off for low contribution: 90.0%. Average assemblages’ dissimilarity – 78.6%. Years 2012 2011 Species Selaroides leptolepis Abundance, N 1.22 Abundance ,N 11.68 Dissimilarity, % 17.67 Contribution, % 22.47 Leiognathus sp. 0 3.98 6.76 8.6 Pentapodus porosus 3.61 4.03 6.75 8.59 Zabidius novemaculeatus 1.22 3.92 6.52 8.29 Pantolabus radiatus 0.83 2.22 3.64 4.63 Paramonacanthus choirocephalus 1.33 0.19 2.83 3.6 Lagocephalus sceleratus 0.67 0.46 1.75 2.22 Caranx sp. 0.39 0.71 1.56 1.99 Choerodon cephalotes 0.72 0.22 1.47 1.87 Choerodon vitta 0.5 0.33 1.43 1.82 Choerodon schoenleinii 0.72 0.06 1.41 1.8 Carcharhinus dussumieri 0.61 0.28 1.3 1.65 Goby 0.67 0.33 1.28 1.63 Echeneis naucrates 0.56 0.33 1.27 1.62 Sillago sp. 0.56 0.19 1.25 1.59 Ambassis sp. 0 1.39 1.18 1.5 Scomberomorus queenslandicus 0.61 0.61 1.16 1.48 Nemipterus hexodon 0.28 0.5 1.09 1.38 Chaetodontoplus duboulayi 0.33 0.33 0.98 1.25 Seriolina nigrofasciata 0 0.54 0.97 1.24 Gnathanodon speciosus 0.22 0.36 0.94 1.19 Choerodon cyanodus 0.17 0.44 0.9 1.15 Flounder 0.33 0.25 0.82 1.04 Monacanthus chinensis 0.39 0.17 0.81 1.02 Anacanthus barbatus 0 0.25 0.79 1 Nemipterus sp. 0 0.34 0.7 0.9 Sillago sihama 0 0.42 0.67 0.85 Carangoides sp. 0 0.42 0.66 0.84 Gymnocranius elongatus 0.17 0.31 0.65 0.83 Lutjanus russelli 0.28 0.11 0.62 0.78 50 Chelmon müelleri 0.11 0.28 0.54 0.69 Carangoides hedlandensis 0.06 0.33 0.54 0.69 51 4. DISCUSSION Comparing results of different analyses in 2011 and 2012 BRUVS survey in Darwin Harbour has enabled a conclusion that observed temporal changes in abundance of some fish species in monitored fish assemblages were mainly related to variations in amounts of two ecological groups of fish: a) pelagic school and non-school fast moving fish and b) demersal open bottom fish. These groups dominated both reefs and open bottom areas at monitoring sites. Pelagic fishes are represented in Darwin Harbour by several species of trevallies, scads and School Mackerel; demersal open bottom fishes include threadfin breams and ponyfishes. Both groups were particularly abundant at “outer” part of the Harbour where open bottom habitat (sand, silted sand and silt with very scarce rocks, algae and macro epibenthos) dominated. Open bottom is a habitat providing limited protection from predators. Small and medium size fishes that live here are school species, those coordinated manner of swimming provide relative protections from solitary predators, enhance foraging success, reproductive advances, helping to navigate in diel movements and seasonal migrations (Breder 1967, Radakov 1973, Shaw 1978, Parrish, Viscedo, & Grunbaum 2002, Steele & Anderson 2006, Travers et al. 2010). Other fishes inhabiting open bottom areas are large mid-water, demersal or benthic predators – trevallies, mackerels, sharks and rays. Most open bottom inhabitants are nomadic or have large home ranges and are constantly roving through habitat searching for food sources: phyto- and zooplankton, bottom epibenthos or fish for predatory species. After food resources in local area have depleted fish are moving away. Changes in fish species richness, population density biomass are common, therefore fish communities in open bottom areas are very dynamic (Hubbs 1974, Yazhi 1982, Ambrose & Meffert 1999, Letourneur et al. 2001, Allen et al. 2006). Our underwater video observations confirmed that fish abundance at monitoring station could change dramatically within several minutes because of arrival or departure of the school of trevally or scad. Variability in fish numbers is not restricted to open bottom areas. More short, but regular movement are typical for some residents of rocky reefs utilising open bottom areas and sea grass beds attracted by food sources available here (Hobson 1972, Robblee & Zieman 1984, Gomelyuk et al. 1985, Hobson & Chess 1986, Lowry & Suthers 1998, Unsworth et al. 2007, Chateau & Wantiez 2009). There is also another important basis for natural variations in small and medium size school fish abundance. Majority of them are very fast growing and short living and their population numbers are prone to frequent and wide fluctuations in their numbers - “waves of life”, typical 52 for organisms with r-strategy (Chetverikov 1915, Timofeev-Resovskii 1958, Pianka 1970, McFarlane & Beamish 2001). In a review done on life history traits and population regulation in marine fishes Kawasaki (1980, 1983) suggested that the grouping of life history traits of marine fishes is more complex than the traditional r and K strategists concept developed for terrestrial animals (MacArthur & Wilson 1967). A quantitative approach to develop grouping of life history strategies in fishes lead to identifying next three endpoint strategies: (1) small, rapidly maturing, short-lived fishes (opportunistic strategists); (2) larger, highly fecund fishes with longer life spans (periodic strategists); and (3) fishes of intermediate size that often exhibit parental investment and produce fewer, larger offspring (equilibrium strategists) (Winemiller & Rose 1992, McCann & Shuter 1997, King & McFarlane 2003). Small school pelagic trevallies and benthic threadfin breams and ponyfishes dominated fish assemblages at both “outer” and “inner” monitoring stations, reef and open bottom habitats belongs to “opportunistic strategists”. Such species have a shorter generation time, fast rate of population growth, despite low individual fecundity (King & MacFarlane 2003). “Opportunistic strategists” occupy habitats with a high degree of variability of biotic and abiotic factors, but potentially, with large food resources (epibenthos, zooplankton). Therefore, their population responses tend to be large in amplitude and species grouped according to this life history strategy have been classified as having either cyclical, irregular or spasmodic population patterns (Caddy & Gulland 1983; Kawasaki 1983; Spencer & Collie 1997). This may result in quick and frequent changes in fish abundance, increasing variability in structure and composition of fish assemblages at open bottom areas. Our data supporting this assumption: notable changes in assemblages both at open sandy/silty bottom at “outer” and “inner” part of the Harbour were due to “opportunistic strategists” - small school trevallies, scads, threadfin breams and pony fishes. Still, because opportunistic strategists are abundant and common species, they are significant part of in inshore ecosystems, playing an important role in food-web as consumers and as a forage fish for predatory fish and dolphins (Parra & Jedensjö 2013). Interpretation of results of monitoring when these species is intricate: on the one hand, there is no need for the immediate alarm if temporal decline in these species was recorded. On the other hand, a persistent decline in such species for several years may be an indicator of possible serious changes in ecosystem leading to an “cascading reaction” in the future, and therefore should not be taken lightly. Because of relatively high population numbers, data “opportunistic strategists” can be analysed using powerful statistical tools. 53 Contrary to the above group, “periodic strategists” are medium to large size slow-growing, long-lived species. They have a lower degree of variability in abundance and have been classified as having a steady-state population pattern (Caddy & Gulland 1983, King & MacFarlane 2003). In Darwin Harbour this group is represented by snappers (family Lutjanidae), gropers (family Serranidae) and mackerels (Scombridae). Longevity (i.e. lifespan greater than 20 years) benefits these species by ensuring a relatively long reproductive cycle, which minimizes the risk that periods of unfavourable environmental conditions that will result in the loss of a stock (Edwards 1984, Leaman & Beamish 1984, Ralston 1987), Newman et al. 2000). Skates and larger sharks belong to “equilibrium strategists” (King & MacFarlane 2003). Both “periodic” and “equilibrium strategists” require very careful monitoring. Despite of their protection from the loss of stocks during the period of unfavourable environmental conditions, their recruitment is not constant and they are very vulnerable to anthropogenic impact – from fishing pressure to habitat loss (King & MacFarlane 2003). Unfortunately, these species have relatively low numbers in collected data for a robust statistical analysis. A usefulness of different indices and statistical analyses method for fish biodiversity monitoring. Different statistical analyses produce a range of metrics that may indicate different significance level of a test. In order to understand what indices identify a meaningful and significant change for monitoring, it is important to differentiate between statistical significance and biological significance. Statistical significance relies on probability and is influenced by sample size. Thus, even trivial changes (from a biological perspective) can be judged to be statistically significant if the sample size is large enough. Therefore, it is important to be able to identify something biologically significant that represents a major shift in fish abundance, assemblage structure and biodiversity. A statistical significant change is not always biologically significant, but a biologically significant change must also be statistically significant. Eventually, the power of a statistical comparison is the ability to detect a biologically meaningful change (Fairweather 1991). Ecological monitoring data is collected from small area, only miniscule fraction of entire area of population distribution. Interpretation of the results should be able to discriminate between “a genuine” fish decline and changes caused by fish re-distribution during diel and seasonal movement, ontogenetic habitat shift (Allen, Pondella II and Horn 2006), local elimination of the portion of population by predators, die off of some individuals of short-living species. The later changes are common in “opportunistic strategists” populations (Lowe-McConnell 1987, King & MacFarlane 2003), particularly in high mobile species like school trevallies, scads, 54 and ponyfishes. Quite often, such changes within a single monitoring station are local phenomenon, not based on (and not reflecting) significant changes in entire population. Decrease in abundance of “opportunistic strategists” may lead to notable changes in fish assemblage composition and structure at some monitoring. However, if “periodic” and “equilibrium” strategists have not been affected and if such changes were recorded only at the limited number of monitoring stations chances what adverse factor are operating in the environment are low. Contrary, acute or chronic increase of adverse factors, both environmental and anthropogenic generally can affect sensitive species from opportunistic, periodic and equilibrium strategists. The impact can manifest themselves in different ways, from elimination of sedentary species due to the habitat degradation and loss to mobile species move from unfavourably altered environment (Leach et al. 1977, Jeff et al. 2001, Launois et al. 2011). The important task of discrimination between these two types of changes is difficult (Whitfield & Elliott 2002). This task become particularly challenging if information on life history and “natural” variability of monitored object is absent or very limited and this is exactly the case for fish assemblages in Northern Australia. There were no major disagreements in results of employed different types of statistical analysis. However, univariate analysis appears to be less sensitive and has provided very limited insight on what actually caused changes in monitored fish assemblages because only one dependent variable being analysed. Multivariate analyses applied to the same set of data were more sensitive and informative, enabling to find that species contributed to detected changes in monitored assemblages. Thus, relying solely on univariate analysis may lead to making type II error while an entire relying on results of multivariate analysis based on assessment of dissimilarity between assemblages may lead to type I error in fish assemblages monitoring. Therefore, the result, of different types of comparison can be interpreted only in conjunction. Changes in fish assemblages at monitoring station in Darwin Harbour in 2011-2012. There were no major noticeable changes in environmental and anthropogenic adverse factors in monitored parts Darwin Harbour environment in 2011-2012 (Darwin Harbour Region Report Cards 2011, 2012). Therefore, it appears, that observed annual differences in fish communities are the result of fish re-distribution rather than a result of decline in fish abundance and biodiversity. Small school pelagic and demersal species that belong to “opportunistic strategists” group (trevallies, threadfin breams and ponyfishes) are major contributors to fish assemblage variability. Natural fluctuations in their abundance can cause swift and often unpredictable changes in fish assemblages structure and abundance. Indeed, 55 these fluctuations should not be assumed as an “information noise” and therefore excluded from monitoring. BRUVS monitoring stations in Darwin Harbour cover a substantial area; thus, it is possible to distinguish between genuine changes in these species abundance and their re-distribution. Further monitoring can provide an important information on temporal and spatial variability in these fishes abundance and natural variations in local fish assemblages. Cooperatively with other types of monitoring (water quality, etc.) obtained information can be used as an important indicator for assessment of changes resulted from anthropogenic pressure on Darwin Harbour ecosystems. 5. Acknowledgements Many people contributed to making this study possible. Larrakia Rangers, Yolande Alley and Yolande Alley participated in a boat trips and have helped with all their effort and enthusiasm. Robyn Henderson from Water Resources was very helpful in coordinating our cooperation with Larrakia Rangers. Division Department of Resources – Fisheries provided the boat. Nathan Crofts, Wayne Baldwin and Poncie Kurnoth from Department of Resources – Fisheries were boat skippers and their contribution was invaluable for safe marine operation and data collection. Daniel Low Choy provided logistic support. I am particularly thankful to Tony Griffiths who provided extensive editions and comments to the draft. 6. References Allen L.G., Yoklavich M. M., Gregor M. Cai Lliet, and M. H. Horn, Chapter 5, Bays and Estuaries, p. 119-148. In: The Ecology of Marine Fishes: California and Adjacent Waters. 2006. L.G. Allen, D.J. Pondella, and M. H. Horn (eds.). University of California Press, Berkeley, 670 pp. Chapter 16 Predation Mark A. Steele And Todd W. Anderson, p. 428-448. In: The Ecology of Marine Fishes: California and Adjacent Waters. 2006. L.G. Allen, D.J. Pondella, and M. H. Horn (eds.). University of California Press, Berkeley, 670 pp. Ambrose R.F., and D. J. Meffert. 1999. Fish-assemblage dynamics in Malibu Lagoon, a small, hydrologically altered estuary in southern California. Wetlands 19:327–340. Anderson M. Gorley R.N. and R. K. Clarke .2008. Permanova+ for Primer: Guide to Software and Statisticl Methods. Plimouth, UK. Breder C. M., Jr. 1967. On the survival value of fish schools. Zoologica, 52: 25–40. Caddy J.F. & Gulland J.A. 1983. Historical patterns of fish stocks. Marine Policy, 7, 267–278. Cappo M., Harvey E., Malcolm H. and Speare P. 2003 Potential of video techniques to monitor diversity, abundance and size of fish in studies of marine protected areas. In Beumer J.P., Grant A. And Smith D.C. (eds) Aquatic protected areas: what works 56 best and how do we know? World Congress on Aquatic Protected Areas Cairns, Australia. Sydney: Australian Society for Fish Biology: 455–464. Cappo M., Speare P. and De’Ath G. 2004 Comparison of baited remote underwater video stations BRUVS and prawn shrimp trawls for assessments of fish biodiversity in inter-reefal areas of the Great Barrier Reef Marine Park. Journal of Experimental Marine Biology and Ecology, 302: 123–152. Cappo M., Stowar M., Syms C., Johansson C. and Cooper T. 2011. Fish-habitat associations in the region offshore from James Price Point – a rapid assessment using Baited Remote Underwater Video Stations (BRUVS). Journal of the Royal Society of Western Australia, 94: 303–321. Cappo M., Harvey E. and Shortis E.M. 2007 Counting and measuring fish with baited video techniques—an overview. In Lyle J.M., Furlani D.M. and Buxton C.D. (eds) Proceedings of the 2006 Australian Society for Fish Biology Conference and Workshop Cutting-edge technologies in fish and fisheries science, Hobart, August 2006. Hobart:ASFB, pp. 101–114. Chateau O, & L Wantiez. 2009. Movement patterns of four coral reef fish species in a fragmented habitat in New Caledonia: implications for the design of marine protected area networks. – ICES Journal of Marine Science, 66: 50–55. Clarke K.R. and Gorley R.N. 2001. PRIMER v5: User manual/tutorial, PRIMER-E. Plymouth UK, 91. Clarke K.R. and Warwick R.M. 2001. Change in marine communities: an approach to statistical analysis and interpretations. Plymouth: Plymouth Marine Laboratory and Natural Environment Research Council. Darwin Harbour Region Report Card 2011, Report No 17/2011D. Department of Natural Resources, Environment, The Arts and Sport. Darwin Harbour Region Report Card 2012, Report No. 18/2012D. Department of Land Resources Management. Dorman S.R, Harvey E.S, Newman S.J. 2012. Bait Effects in Sampling Coral Reef Fish Assemblages with Stereo-BRUVs. Public Library of Science One, 7(7). Faul F., Erdfelder, E., Lang, A.-G., and A Buchner. 2007. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods, 39: 175-191. Friedlander A. M., E. K. Brown, P. L. Jokiel, W. R. Smith, and K. S. Rodgers. 2003. Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs, 22: 291–305. Friedlander A. M, and J. D Parrish. 1998. Journal of Experimental Marine Biology and Ecology, 224, 1: 1-30. 57 Fairweather P.G. 1991. Statistical Power and Design Requirements for Environmental Monitoring. Australian Journal of Marine and Fresh water Research, 42: 555-567. Gomelyuk V. 2008. Fish assemblages composition and structure in three shallow habitats in north Australian tropical bay, Garig Gunak Barlu National Park, Northern Territory, Australia. Journal of the Marine Biological Association of the United Kingdom, 89, 3: 449–460. Gomelyuk V. E., Markevich A. I., and V. P. Leunov. 1985. Diurnal Rhythm of the Activity of Eastern Rockfish in Peter the Great Bay of the Sea of Japan. Russian Journal of Marine Biology, 3: 68–71. Harvey E.S., Newman, S.J. McLean, D.L., Cappo, M., Meeuwig, J.J. and C.L. Skepper. 2012. Comparison of the relative efficiencies of stereo-BRUVs and traps for sampling tropical continental shelf demersal fishes. Fisheries Research, 125–126: 108–120. Hobson E. S. 1972. Activity of Hawaiian reef fishes during the evening and morning transitions between daylight and darkness. Fisheries Bulletin, 70: 715-740. Hobson E.S. and J.R. Chess. 1986. Relationships among fishes and their prey in a nearshore sand community off southern California. Environment Biology Fishes, 17: 201–226. International Code of Zoological Nomenclature (1999). International code of zoological nomenclature adopted by the International Union of Biological Resources Anderson, M.J. 2001a. A new method for non-parametric multivariate analysis of variance. Australian Ecology, 26: 32-46. Jeff T. Tyson, Roger L. Knight. 2001. Response of Yellow Perch to Changes in the Benthic Invertebrate Community of Western Lake Erie. Transactions of the American Fisheries Society,130 (5): 766–782. Kawasaki T. 1980. Fundamental relations among the selections of life history in marine teleosts. Bulletin of the Japanese Society of Science and Fisheries, 46: 289–293. Kawasaki T. 1983. Why do some pelagic fishes have wide fluctuations in their numbers? Biological basis of fluctuation from the viewpoint of evolutionary ecology. FAO Fisheries Report, 291: 1065–1080. King J.R. and G.A. McFarlane. 2003. Marine fish life history strategies: applications to fishery management. Fisheries Management and Ecology, 10: 249–264. Larry Glenn Allen, Daniel Pondella II, and Michael H. Horn. 2006. Ecology of marine fishes : California and adjacent waters. Berkeley: University of California Press. Leaman, B.M., and R.J. Beamish. 1984. Ecological and management implications of longevity in some northeast Pacific groundfishes. International Fisheries Commission Bulletin, 42: 85-97. 58 Leach J. H., Johnson M. G., Kelso J. R. M., Hartmann J., Nümann W., and B. Entz. 1977. Responses of Percid Fishes and Their Habitats to Eutrophication. Journal of the Fisheries Research Board of Canada, 34(10): 1964-1971. Letourneur Y., Darnaude A., Salen-Picard C., Harmelin-Vivien M. 2001. Spatial and temporal variations of fish assemblages in a shallow Mediterranean soft-bottom area (Gulf of Fos, France). Oceanologica Acta. 24:273-285. Lionel Launois, Jacques Veslot, Pascal Irz, Christine Argillier. 2011. Development of a fishbased index (FBI) of biotic integrity for French lakes using the hindcasting approach. Ecological Indicators, 11 (6): 1572 – 1583. Lowry M. B. and I. M. Suthers. 1998. Home range, activity and distribution patterns of a temperate rocky-reef fish, Cheilodactylus fuscus. Marine Biology, 132, 4, pp 569578. Lowe-McConnell R.H. 1987. Ecological studies in tropical fish communities. Cambridge, Press Syndicate of the University of Cambridge. MacArthur, R., Wilson, E.O. 1967. The Theory of Island Biogeography 2001 (reprint ed.). Princeton University Press. McCann K. and Shuter B.J. 1997 Bioenergetics of life history strategies and the comparative allometry of reproduction. Canadian Journal of Fisheries and Aquatic Sciences, 54: 1289–1298. McClanahan and T.R., R. Arthur. 2001. The effect of marine reserves and habitat on populations of East African coral reef fishes. Ecological Applications, 11(2): 559569. McFarlane G.A. and Beamish R.J. 2001 The re-occurrence of sardine off British Columbia characterises the dynamic nature of regimes. Progress in Oceanography, 49: 151– 165. Morrisey D.J. Underwood A.J. Howitt and J.S. Stark 1992. Temporal variations in softsediment benthos. Marine Ecology Progress Series, 81: 197-204. National Marine Fisheries Service (NMFS). 1999. Ecosystem- Based Fishery Management. A Report to Congress by the Ecosystem Principles Advisory Committee. United States Department of Commerce, National Oceanic and Atmospheric Administration, NMFS, Silver Springs, Maryland, 54 pp. Newman, S. J., Steckis, R.A., Edmonds, J. S. and Lloyd, J. 2000. Stock structure of the goldband snapper (Pristipomoides multidens) (Pisces: Lutjanidae) from the waters of northern and western Australia by stable isotope ratio analysis of sagital otolith carbonate. Marine Ecology Progress Series, 198: 239-247. Newman S.J., Cappo M. and D.M.B. Williams. 2000. Age, growth, mortality rates and corresponding yield estimates using otoliths of the tropical red snappers, Lutjanus 59 erythropterus, L. malabaricus and L. sebae, from the central Great Barrier Reef. Fisheries Research, 48 (1): 1-14. Ommaney F.D. 1963. The Fishes. (Life Nature Library) New York: Time Incorporated. Parra G.J. and Jedensjö M. 2013. Stomach contents of Australian snubfin (Orcaella heinsohni) and Indo-Pacific humpback dolphins (Sousa chinensis). Marine Mammals Science, DOI: 10.1111/mms.12088. Parrish J. K.; Viscedo, S. C.; Grunbaum, D. 2002. Self organised fish-schools: An examination of emergent properties. Biological Buletin, 202 (3): 296–305. Pianka E.R. 1970. On r and K selection. American Naturalist, 104 (940): 592–597. Priede I.G, Merrett N.R (1996) Estimation of abundance of abyssal demersal fishes; a comparison of data from trawls and baited cameras. Journal of Fish Biology, 49 (Suppl A): 207–216. Ralston S. 1987. Mortality rates of snappers and groupers. In: Tropical Snappers and Groupers: Biology and Fisheries Management, J.J.Polovina and S. Ralston (eds), pp. 505-532. Radakov D.V. 1973. Schooling in the ecology of fish. Israel Program for Scientific Translation, translated by Mill H. Halsted Press, New York. Robblee M.B. and J.C. Zieman. 1984. Diel variation in the fish fauna of a tropical seagrass feeding ground. Bulletin of Marine Science, 34(3): 335-345. Roberts C. M and R.F.G. Ormond. 1987. Habitat complexity and coral reef fish diversity and abundance on Read Sea fringing reefs. Marine Ecology Progress Series, 41: 1-8. Shaw E. 1978. Schooling fishes. American Scientist, 66: 166–175. Soto-Galera, E., E. Díaz-Pardo, E. López-López, and J. Lyons. 1998. Fishes as indicators of environmental quality in the Río Lerma basin, México. Aquatic Ecosystem Health and Management, 1: 267-276. Spencer P.D. and Collie J.S. 1997 Patterns of population variability in marine fish stocks. Fisheries Oceanography 6, 188–204. Timofeev-Resovskii, N. V. Mikroevoliutsiia, elementarnye iavleniia, material i faktory mikroevoliutsionnogo protsessa.” Botanicheskii zhurnal, 1958: 43, 3: 27-35. Tolimieri N. 1995. Effects of microhabitat characteristics on the settlement and recruitment of a coral reef fish at two spatial scales. Oecologia, 102: 52-63. Tolimieri N. 1998. Effects of substrata, resident conspecifics and damselfish on the settlement and recruitment of the stoplight parrotfish, Sparisoma viride. Environmental Biology of Fishes, 53: 393-404. Travers M. J., Potter I. C., Clarke K. R., Newman S. J. and J. B. Hutchins. 2010. The inshore fish faunas over soft substrates and reefs on the tropical west coast of Australia 60 differ and change with latitude and bioregion: Ichthyofaunas of soft substrates and reefs. Journal of Biogeography, 37, 1: 148-169. Unsworth R.K.F., Bell J.J. and D.J. Smith 2007. Tidal fish connectivity of reef and sea grass habitats in the Indo-Pacific. Journal of Marine Biological Association of United Kingdom, 87: 1287–1296. Watson D.I., Harvey E.S. Anderson M.J., Kendrick G.A. 2005. Marine Biology, 148: 415-425. Waves of life (Observations of Lepidoptera in the summer of 1903). 1905. Izvestiia Imperatorskogo obshchestva liubitelei estestvoznaniia, antropologii i etnografii, 98 , Dnevnik zoologicheskogo otdeleniia, 3 , 6: 106–110. Willis T.J, Babcock R.C. 2000 A baited underwater video system for the determination of relative density of carnivorous reef fish. Marine & Freshwater Research, 51:755– 763. Winemiller K.O. and Rose K.A. 1992. Patterns of life-history diversification in North American fishes: implications for population regulation. Canadian Journal of Fisheries and Aquatic Sciences, 49: 2196–2218. Yazhi Z.Q.Z. 1982. Preliminary Study on Seasonal Changes of Species Compositions of Demersal Fishes in South-FujianTaiwan Bank Fishing Ground. Journal of Xiamen University (Natural Science), 1: 49–55. 61 7. APPENDIX Table 12. Post hoc power analysis of F tests one-way ANOVA of values of mean fish species number at North-West Channel Island site in Darwin Harbour, stations 1-NCHB, 2-NCHB and 4RCK-NCH in 2011-2012. Input Effect size f 0.515 α err prob 0.050 Total sample size 36 Output Number of groups 6 Noncentrality parameter λ 29.554 Critical F 2.534 Numerator df 5 Denominator df 30 Achieved power (1-β err prob) 0.964 Table 13. Post hoc power analysis of F tests one-way ANOVA of values of mean MaxN at NorthWest Channel Island site in Darwin Harbour, stations 1-NCHB, 2-NCHB and 4RCK-NCH in 20112012. Input Effect size f 1.141 α err prob 0.05 Total sample size 36 Output Number of groups 6 Noncentrality parameter λ 46.899 Critical F 2.533 Numerator df 5 Denominator df 30 Power (1-β err prob) 0.999 62 Table 14. Post hoc power analysis of F tests one-way ANOVA of values of mean fish species number at The Entrance site in Darwin Harbour, stations DSAC-B, 8-RCK and 6-RCK in 20112012. Input Effect size f 0.715 α err prob 0.05 Total sample size 36 Output Number of groups 6 Noncentrality parameter λ 18.427 Critical F 2.534 Numerator df 5 Denominator df 30 Power (1-β err prob) 0.875 Table 15. Post hoc power analysis of F tests one-way ANOVA of values of mean MaxN at The Entrance site in Darwin Harbour, stations DSAC-B, 8-RCK and 6-RCK in 2011-2012. Input Effect size f 0.993 α err prob 0.05 Total sample size 36 Output Number of groups 6 Noncentrality parameter λ 29.588 Critical F 2.620 Numerator df 5 Denominator df 30 Power (1-β err prob) 0.978 63 Table 16. Post hoc power analysis of F tests one-way ANOVA of values of mean fish species number at Rick Mills artificial reef site in Darwin Harbour, stations RM-1, RM-2 and RM-3 in 2011-2012. Input Effect size f 0.867 α err prob 0.05 Total sample size 36 Output Number of groups 6 Noncentrality parameter λ 27.065 Critical F 2.533 Numerator df 5 Denominator df 30 Power (1-β err prob) 0.973 Table 17. Post hoc power analysis of F tests one-way ANOVA of values of MaxN at Rick Mills artificial reef site in Darwin Harbour, stations RM-1, RM-2 and RM-3 in 2011-2012. Input Effect size f 0.8152 α err prob 0.05 Total sample size 36 Output Number of groups 6 Noncentrality parameter λ 23.926 Critical F 2.533 Numerator df 5 Denominator df 30 Power (1-β err prob) 0.951 64 Table 18. Post hoc power analysis of F tests one-way ANOVA of values of mean fish species number at Bottle Washer artificial reef site in Darwin Harbour, stations BW-1, BW-2 and BW-3 in 2011-2012. Input Effect size f 0.740 α err prob 0.05 Total sample size 36 Output Number of groups 6 Noncentrality parameter λ 19.733 Critical F 2.533 Numerator df 5 Denominator df 30 Power (1-β err prob) 0.899 Table 19. Post hoc power analysis of F tests one-way ANOVA of values of MaxN at Bottle Washer artificial reef site in Darwin Harbour, stations BW-1, BW-2 and BW-3 in 2011-2012. Input Effect size f 0.815 α err prob 0.05 Total sample size 36 Output Number of groups 6 Noncentrality parameter λ 23.926 Critical F 2.533 Numerator df 5 Denominator df 30 Power (1-β err prob) 0.951 65 Table 20. ANOVA of number of fish species in one 1-hour BRUVS video sample, pooled data of 12 monitoring stations in Darwin Harbour in 2011-2011. Source Outer-Inner stations*Years Error Type III SS 866.805 1,136.035 df 3 152 Mean Squares 288.035 7.474 F-Ratio 38.659 p-Value 0.000 Table 21. ANOVA of number of fish in one 1-hour BRUVS video sample (MaxN), pooled data of 12 monitoring stations in Darwin Harbour in 2011-2011. Source Outer-Inner stations*Years Error Type III SS 16,655.660 33,871.333 df 3 152 Mean Squares 5,551.887 222.838 F-Ratio 24.914 p-Value 0.000 Table 22. ANOVA of mean fish number (MaxN) in BRUVS samples at three monitoring stations at North-West site of Channel Island in Darwin Harbour in 2011-2012. Source Stations *Years Error Type III SS 2,103.000 1,971.000 df 11 60 Mean Squares 191.182 32.850 F-Ratio 5.820 p0.000 Value Table 23. ANOVA of mean fish species number (FSN) in BRUVS samples at three monitoring stations at North-West site of Channel Island in Darwin Harbour in 2011-2012. Source Stations *Years Error Type III SS 25.139 95.833 df 5 30 Mean Squares 5.028 3.194 F-Ratio 1.574 p0.198 Value Table 24. ANOVA of mean fish number (MaxN) in BRUVS samples at three monitoring stations at Entrance site, Darwin Harbour in 2011-2012. Source Stations *Years Type III SS 1,112.472 df 5 Mean Squares 222.494 F-Ratio 5.042 p0.002 Value 66 Error 1,323.833 30 44.128 Table 25. ANOVA of mean mean fish species number (FSN) in BRUVS samples at three monitoring stations at the Entrance site, Darwin Harbour in 2011-2012. Source Stations Error *Years Type III SS 71.889 138.333 df 5 30 Mean Squares 14.378 4.611 F-Ratio 3.118 p0.022 Value Table 26. ANOVA of mean fish number (MaxN) in BRUVS samples at three monitoring stations at Rick Mills artificial reef site, Darwin Harbour in 2011-2012. Source Stations Error *Years Type III SS 5,700.250 10,516.500 df 5 30 Mean Squares 1,140.050 350.550 F-Ratio 3.252 p-Value 0.018 Table 27. ANOVA of mean mean fish species number (FSN) in BRUVS samples at three monitoring stations at Rick Mills artificial reef site,Darwin Harbour in 2011-2012. Source Stations Error *Years Type III SS 176.472 202.500 df 5 30 Mean Squares 35.294 6.750 F-Ratio 5.229 p-Value 0.001 Table 28. ANOVA of mean fish number (MaxN) in BRUVS samples at three monitoring stations at Bottle Washer artificial reef site, Darwin Harbour in 2011-2012. Source Stations Error *Years Type III SS 8,075.793 5,972.688 df 5 30 Mean Squares 1,615.159 199.090 F-Ratio 8.113 p-Value 0.001 67 Table 29. ANOVA of mean mean fish species number (FSN) in BRUVS samples at three monitoring stations at Bottle Washer artificial reef site,Darwin Harbour in 2011-2012. Source Stations Error *Years Type III SS 264.333 486.667 df 5 30 Mean Squares 52.867 16.222 F-Ratio 3.259 p-Value 0.018 68