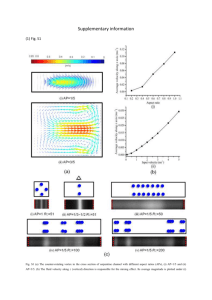
ROTAXINATED OPALS revised SI
advertisement

SUPPORTING INFO FOR: Fluorescent polystyrene photonic crystal self-assembled with water-soluble conjugated polyrotaxanes Francesco Di Stasio,1,2 Luca Berti,3 Shane O. McDonnell,4 Valentina Robbiano,1 Harry L. Anderson,4 Davide Comoretto3 and Franco Cacialli1* 1 Department of Physics and Astronomy, and London Centre for Nanotechnology, University College London, Gower Street, London, WC1E 6BT, United Kingdom 2 Current address: Istituto Italiano di Tecnologia, Via Morego 30, IT-16163, Genoa, Italy 3 Dipartimento di Chimica e Chimica Industriale, Università degli Studi di Genova, Via Dodecaneso 31, Genova, 16146, Italy 4 Chemistry Research Laboratory, Department of Chemistry, University of Oxford, Mansfield Road, Oxford, OX1 3TA, United Kingdom * Author to whom correspondence should be addressed. Electronic mail : f.cacialli@ucl.ac.uk Fig. SI1. Reflectance spectra of 5 different opal films incorporating polyrotaxanes fabricated with d = 222 nm (a,b,c and d) and d = 200 nm (e) polystyrene nanospheres. Reflectance spectra were collected in different areas of the samples to control the uniformity of the opal films and their optical properties. The quality of the opal structure is preserved upon polyrotaxanes incorporation as demonstrated by the presence in the optical spectra (for wavelengths below 300 nm) of the van Hove like structures (Fig. SI1). These features are due to the diffraction of light along directions different form the incident one 1 and are known to strongly depend on both the order of the system1 and on the microsphere quality.2 The presence of van Hove like structures is observed in different opal films fabricated with 222 nm nanospheres (Fig. S1a-d) or 200 nm (Fig. S1e) confirming the high reproducibility of the fabrication procedure employed. Furthermore, only minor changes in reflectance peak position and shape are observed in all opal films thus testifying the good uniformity of the incorporation process. In fact, such minor changes are usually observed in bare opals as well.1-5 50 Transmittance (%) 40 0° 4° 8° 12° 16° 20° 24° 28° 32° 36° 40° 30 20 10 0 300 350 400 450 500 550 Wavelength (nm) Fig. SI2. Transmittance spectra of a 200 nm opal incorporating polyrotaxanes as a function of the incidence angle. Transmittance (%) 40 40° 36° 32° 28° 24° 20° 16° 12° 8° 4° 0° 30 20 10 0 300 350 400 450 500 550 Wavelength (nm) Fig. SI3. Transmittance spectra of a 222 nm opal incorporating polyrotaxanes as a function of the incidence angle. van Hove-like structures in opals show dispersion properties (dependence on the incidence angle, i.e. on the photon wavevector) opposite to that of the pseudo-gap.1,2 To assess the quality of our infiltrated opals, grown by a non-standard technique, we measured the transmittance spectra of 200 and 222 nm opals as a function of the incidence angle (Fig. SI2 and SI3, respectively). Overlapped to the strong and broad polymer absorption, both spectra show two main features: 1) The pseudo gap, observed at ~ 470 and 510 nm for 200 and 222 nm opals at normal incidence respectively, exhibiting a dispersion towards shorter wavelengths upon increasing the incidence angle; 2) The van Hove-like features, observed at ~ 300 nm, exhibiting dispersion towards longer wavelengths upon increasing the incidence angle. These two features are not very pronounced in the spectra since almost buried into the polymer optical absorption and the scattering usually observed for opals in this spectral region.1-6 In spite of that, the relative intensity of the van Hove singularities with respect to the stop band peak as observed in the reflectance spectra as well as the dispersion properties testify very good opal quality. References 1 2 3 4 5 6 E. Pavarini, L. C. Andreani, C. Soci, M. Galli, and F. Marabelli, Phys. Rev. B 72, 045102 (2005). D. Comoretto, V. Robbiano, G. Canazza, L. Boarino, G. Panzarasa, M. Laus, and K. Sparnacci, Polym. Composite. 34 (9), 1443 (2013). J. F. Galisteo-Lopez, M. Ibisate, R. Sapienza, L. S. Froufe-Perez, A. Blanco, and C. Lopez, Adv. Mater. 23, 30 (2011). A. Pasquazi, S. Stivala, G. Assanto, V. Amendola, M. Meneghetti, M. Cucini, and D. Comoretto, Appl. Phys. Lett. 93 (9), 3 (2008). L. Berti, M. Cucini, F. Di Stasio, D. Comoretto, M. Galli, F. Marabelli, N. Manfredi, C. Marinzi, and A. Abbotto, J. Phys. Chem. C 114 (6), 2403 (2010) I. S. Nikolaev, P. Lodahl, and W. L. Vos, Phys. Rev. A 71 (5), 10 (2005).