gbc20220-sup-0002-anthropogenic
advertisement

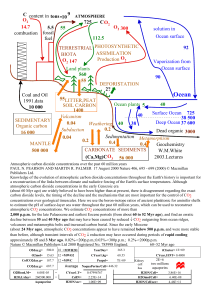
1 Supporting Information for 2 Six centuries of changing oceanic mercury 3 Yanxu Zhang, Lyatt Jaeglé, LuAnne Thompson, David Streets 4 5 1 Model comparison to modern atmospheric mercury observations 6 We compare our simulation of present-day (2008) atmospheric Hg to observations 7 collected in the atmospheric marine boundary layer and at terrestrial sites, as summarized 8 in previous GEOS-Chem model studies [Selin et al., 2007; Holms et al., 2010; Soerensen 9 et al., 2010; Soerensen et al., 2012]. These ground-based observations were collected 10 between 2004 and 2008 at 42 ground-sites. Observations in the marine boundary layer are 11 from 4 cruises over the same period. 12 Figure S1 (top panel) shows the modeled spatial distribution of surface 13 atmospheric total gaseous mercury (TGM = Hg0+HgII) concentrations, while Figure S2 14 shows the latitudinal distribution of TGM concentrations. Our present-day simulation 15 predicts a global mean atmospheric TGM concentration of 1.2 ng/m3. The present-day 16 distribution of TGM shows large spatial variability, with concentrations ranging from 0.5 17 to 3.5 ng/m3. The highest concentrations are modeled over regions with strong 18 anthropogenic emissions including East Asia, Western Europe, and North America 19 (Figure S1 top panel). The mean observed concentrations of TGM are 2.0±0.88 ng/m3, 20 compared to 1.4±0.50 ng/m3 in the model sampled at the location of observations. The 21 spatial distribution of TGM is similar in both model and observations, with higher 1 1 concentrations near strong anthropogenic regions. The spatial correlation coefficients 2 between model and observation is high (r2 = 0.90). For terrestrial sites, the modeled TGM 3 concentrations (1.5±0.5 ng/m3) are 25% lower than observations (1.9±1.0 ng/m3). The 4 model also under-predicts observed TGM concentrations over the North Atlantic (obs.: 5 1.9±0.16 ng/m3; mod.: 1.3±0.02 ng/m3) and North Pacific Oceans (obs.: 2.5±0.4 ng/m3; 6 mod.: 1.3±0.07 ng/m3). 7 Anthropogenic emissions lead to a small interhemispheric gradient, with 1.2-1.5 8 ng/m3 in the northern hemisphere and 0.9-1.0 ng/m3 in the southern hemisphere (Figure 9 S2 top panel). The modeled latitudinal gradient falls at the low edge of the envelope of 10 observations. 11 12 13 2 Model comparison to concentrations of Hg in the surface ocean Figure S1 (bottom panel) shows the modeled present-day total aqueous Hg 14 concentrations in the surface ocean. The latitudinal distribution of zonal average 15 concentrations is shown in Figure S2 (bottom panel). The modeled global mean total Hg 16 concentration over the surface ocean is 0.72 pM, with no significant latitudinal and inter- 17 hemispheric gradients. Overall, the large-scale horizontal spatial pattern of surface ocean 18 Hg concentrations has not changed much at present-day compared with that for natural 19 conditions, indicating similar controlling factors (e.g. ocean circulation and particle 20 sinking flux) [Zhang et al., 2014]. 21 We compare our model results of surface ocean Hg concentrations to observations 22 spanning the last two decades, as summarized by Soerensen et al. [2010]. Observations 23 display a large variability, with values ranging from less than 0.1 pM to more than 7 pM 2 1 (Figure S1 bottom panel). Observed median total Hg concentrations are 1.0 pM. The 2 model sampled at the same locations as the observations displays median values that are 3 33% lower (0.67 pM). The modeled concentrations fall within the center of envelope for 4 the observations with most density data points (Figure S2 bottom panel), indicating a 5 reasonable agreement between them. For the North Atlantic Ocean (obs: 1.0±0.48 pM; 6 mod: 0.68±0.12 pM), North Pacific Ocean (obs: 0.88±0.31 pM; mod.: 0.70±0.11 pM) and 7 Southern Ocean (obs: 1.0±0.44 pM; mod: 0.69±0.12 pM), we find that the model 8 captures observed surface ocean total Hg concentrations reasonably well. 9 Most of the measurements near coastal and shelf regions are significantly higher 10 (> 2 pM) than the corresponding modeled values (~0.5 pM), such as the measurements 11 over the Hudson Bay region and the coastal Canadian Arctic Ocean Archipelago. This 12 could be due to an underestimate of the riverine input Hg flux, especially because we 13 only considered the erosion of Hg from background soils in our model and do not take 14 into account the historical influence of wastewater discharges from commercial sources 15 [Soerensen et al., 2010; Streets et al., 2011; Horowitz et al., 2014] or the impact of 16 snowpack melt water [Fisher et al., 2012]. Furthermore, the global model does not have 17 adequate resolution to capture advection and mixing of Hg near the coasts. 18 3 1 2 3 Figure S1. Comparison of model results with present-day observations. Top panel: 4 Modeled present-day surface concentrations of atmospheric total gaseous mercury (TGM, 5 in ng/m3). Observed concentrations (2004-2008) are shown as color-coded circles 6 (cruises) and diamonds (ground-based sites). Bottom: Total Hg concentrations (in pM) in 7 the surface ocean. Observed concentrations (1984-2010) are shown as color-coded 8 circles. 4 1 2 3 Figure S2. Zonal mean of Hg concentrations in the atmosphere and surface ocean. 4 Zonal average of top) near-surface atmospheric TGM concentrations and bottom) total 5 Hg concentrations at ocean surface. Observations are shown as open circles. The solid 6 lines correspond to the present-day (orange) and pre-anthropogenic (blue) simulations. 7 3 Uncertainty Analysis 8 9 The uncertainty associated with our estimate of anthropogenic Hg in the ocean is primarily from the historical emission of Hg and our parameterization of the 10 biogeochemistry of Hg in the ocean. With and without reducing the Hg mining emissions 11 by a factor of 3 result in the total anthropogenic Hg emissions during 1450-2008 as 950 12 and 1750 Mmol, respectively. Adopting the original inventory causes a 50% increase in 13 the anthropogenic Hg in the present-day ocean. This could be even higher if we were to 14 consider the release of commercial Hg to the environment [Horowitz et al., 2014]. As a 15 lower end, reducing the historical Hg emissions in Au/Ag mining to zero results in the 16 total anthropogenic Hg emission as 550 Mmol, which would cause a 20-30% decrease in 5 1 the anthropogenic Hg load in the present-day ocean. The redox chemistry between Hg0 2 and HgII and the partitioning coefficient (Kd) of dissolved HgII onto the particulate phase 3 also play important roles, because they affect the fraction of total Hg as HgII and the 4 strength of its particle scavenging. Observed fractions of HgII range from 50%-80% in the 5 subsurface waters [Lamborg et al., 2014], leading to an uncertainty range of 50%. 6 Although the Kd value could vary for an order of magnitude based on available 7 observations [Soerensen et al., 2010], sensitivity runs suggest the plausible range for this 8 parameter is within a factor of 2 of our current used value, which leads to an additional 9 40% variation in the amount of anthropogenic Hg in the ocean. Another source of 10 uncertainty comes from the model’s representation of the biological pump itself, 11 especially the remineralization depth of sinking organic particles. Choosing different 12 remineralization depths results in a variation of 30% for the amount of anthropogenic Hg 13 in the ocean. Overall, these factors cause an uncertainty range of 190 – 530 Mmol. 14 6 1 Additional information for observed lake sediment profiles 2 3 Table S1. Detailed information for the source of lake sediment observations shown in Figure 1 Category Remote Eastern U.S. East Asia 4 a Region Greenland Chile West Greenland California-Nevada, U.S. Svalbard Upper Midwest, U.S. Alaska Arctic Ny-Ålesund Brazil New Zealand Western U.S. High-latitude Canada Santa Barbara Basin Western U.S. Washington, U.S. Uganda Upper Midwest, U.S. Vermont and New Hampshire, U.S. Nova Scotia Adirondack Lake, New York, U.S. Maine, U.S. Midcontinental North America Qinghai Lake, Tibetan Plateau Taihu Lake, China Tibetan Plateau na 1 2 3 2 4 3 15 1 4 1 14 4 1 9 1 3 12 10 3 8 1 7 1 2 8 Reference Asmund and Nielsen, 2000 Biester et al., 2002 Bindler et al. 2001 Drevnick et al., 2010 Drevnick et al., 2012 Engstrom and Swain, 1997 Fitzgerald et al., 2005 Jiang et al., 2011 Lacerda et al., 1999 Lamborg et al., 2002 Landers et al., 2008 Lockhart et al., 1995 Young et al., 1973 Mast et al., 2010 Paulson and Norton, 2008 Yang et al., 2010 Engstrom and Swain, 1997 Kamman and Engstrom, 2002 Lamborg et al., 2002 Lorey and Driscoll, 1999 Roos-Barraclough et al., 2006 Swain et al., 1992 Wang et al., 2010 Wu et al., 2008 Yang et al., 2010 Number of lake sediment cores used for each location 5 7 1 References 2 Asmund, G., and S. P. Nielsen (2000), Mercury in dated Greenland marine sediments, 3 Sci. Total Environ., 245(1-3), 61-72. 4 Biester, H., R. Kilian, C. Franzen, C. Woda, A. Mangini, and H. F. Scholer (2002), 5 Elevated mercury accumulation in a peat bog of the Magellanic Moorlands, Chile (53 6 degrees S) - an anthropogenic signal from the Southern Hemisphere, Earth Planet. Sc. 7 Lett., 201, 609-620. 8 Bindler, R., I. Renberg, P. G. Appleby, N. J. Anderson, and N. L. Rose (2001), Mercury 9 accumulation rates and spatial patterns in lake sediments from west Greenland: A coast to 10 ice margin transect, Environ. Sci. Technol., 35, 1736-1741. 11 Drevnick, P. E., A. L. C. Shinneman, C. H. Lamborg, D. R. Engstrom, M. H. Bothner, 12 and J. T. Oris (2010), Mercury Flux to Sediments of Lake Tahoe, California–Nevada, 13 Water Air Soil Poll., 210, 399-407. 14 Drevnick, P. E., H. Yang, C. H. Lamborg, and N. L. Rose (2012), Net atmospheric 15 mercury deposition to Svalbard: Estimates from lacustrine sediments, Atmos. Environ., 16 59, 509-513. 17 Engstrom, D. R., and E. B. Swain (1997), Recent declines in atmospheric mercury 18 deposition in the upper Midwest, Environ. Sci. Technol., 31, 960-967. 19 Fitzgerald, W. F., D. R. Engstrom, C. H. Lamborg, C. M. Tseng, P. H. Balcom, and C. R. 20 Hammerschmidt (2005), Modern and historic atmospheric mercury fluxes in northern 21 Alaska: Global sources and Arctic depletion, Environ. Sci. Technol., 39, 557-568. 8 1 Fisher, J.A., D.J. Jacob, A.L. Soerensen, H.M. Amos, A. Steffen, and E.M. Sunderland 2 (2012), Riverine source of Arctic Ocean mercury inferred from atmospheric 3 observations, Nature Geosci., 5, 499-504. 4 Holmes, C. D., D. J. Jacob, E. S. Corbitt, J. Mao, X. Yang, R. Talbot, and F. Slemr 5 (2010), Global atmospheric model for mercury including oxidation by bromine atoms, 6 Atmos. Chem. Phys., 10(24), 12037-12057. 7 Horowitz, H. M., D. J. Jacob, H. M, Amos, D. G. Streets, and E. M. Sunderland 8 (2014), Historical mercury releases from commercial products: global environmental 9 implications, Env. Sci. Tech., 48(17), 10242-10250. 10 Jiang, S., X. Liu, and Q. Chen (2011), Distribution of total mercury and methylmercury 11 in lake sediments in Arctic Ny-Alesund, Chemosphere, 83, 1108-1116. 12 Kamman, N. C., and D. R. Engstrom (2002), Historical and present fluxes of mercury to 13 Vermont and New Hampshire lakes inferred from Pb-210 dated sediment cores, Atmos. 14 Environ., 36, 1599-1609. 15 Lacerda, L. D., M. G. Ribeiro, R. C. Cordeiro, A. Sifeddine, and B. Turcq (1999), 16 Atmospheric mercury deposition over Brazil during the past 30,000 years, Environ. 17 Biodivers., 51, 363-371. 18 Lockhart, W. L., P. Wilkinson, B. N. Billeck, R. V. Hunt, R. Wagemann, and G. J. 19 Brunskill (1995), Current and historical inputs of mercury to high-latitude lakes in canada 20 and to hudson-bay, Water Air Soil Pollut., 80, 603-610. 21 Lamborg, C. H., W. F. Fitzgerald, A. W. H. Damman, J. M. Benoit, P. H. Balcom, and D. 22 R. Engstrom (2002), Modern and historic atmospheric mercury fluxes in both 9 1 hemispheres: Global and regional mercury cycling implications, Global Biogeochem. 2 Cy., 16, 1104, doi:10.1029/2001GB001847. 3 Lamborg, C., K. Bowman, C. Hammerschmidt, C. Gilmour, K. Munson, N. Selin, and 4 C.-M. Tseng (2014), Mercury in the Anthropocene Ocean, Oceanography, 27(1), 76-87. 5 Landers, D.H., H. E. Tayler, S. Simonich, K. Hageman, D. Jaffe, S. Usenko, L. Geiser, L. 6 Ackerman, D. H. Campbell, J. Schrlau, A. Schwindt, N. Rose, C. Schreck, T. Blett, M. 7 kent, M. M. Erway, W. Hafner (2008), The fate, transport, and ecological impacts of 8 airborne contaminants in western national parks (USA). In: Christie, S. (Ed.), Western 9 Airborne Contaminants Assessment Project Final Report, vol. 1 EPA/600/R-07/138. 10 Lorey, P., and C. T. Driscoll (1999), Historical trends of mercury deposition in 11 Adirondack lakes, Environ. Sci. Technol., 33, 718-722. 12 Mast, M. A., D. J. Manthorne, and D. A. Roth (2010), Historical deposition of mercury 13 and selected trace elements to high-elevation National Parks in the Western U.S. inferred 14 from lake-sediment cores, Atmos. Environ., 44, 2577-2586. 15 Paulson, A. J., and D. Norton (2008), Mercury Sedimentation in Lakes in Western 16 Whatcom County, Washington, USA and its Relation to Local Industrial and Municipal 17 Atmospheric Sources, Water Air Soil Pollut. 189, 5-19. 18 Roos-Barraclough, F., N. Givelet, A. K. Cheburkin, W. Shotyk, and S. A. Norton (2006), 19 Use of Br and Se in peat to reconstruct the natural and anthropogenic fluxes of 20 atmospheric Hg: A 10000-year record from Caribou Bog, Maine, Environ. Sci. Technol. 21 40, 3188-3194. 10 1 Selin, N. E., D. J. Jacob, R. J. Park, R. M. Yantosca, S. Strode, L. Jaegle, and D. Jaffe 2 (2007), Chemical cycling and deposition of atmospheric mercury: Global constraints 3 from observations, J. Geophys. Res., 112(D2), D02308, doi: 10.1029/2006JD007450. 4 Soerensen, A. L., E. M. Sunderland, C. D. Holmes, D. J. Jacob, R. M. Yantosca, H. Skov, 5 J. H. Christensen, S. A. Strode, and R. P. Mason (2010), An Improved Global Model for 6 Air-Sea Exchange of Mercury: High Concentrations over the North Atlantic, Environ. 7 Sci. Technol., 44(22), 8574-8580. 8 Soerensen, A. L., D. J. Jacob, D. G. Streets, M. L. I. Witt, R. Ebinghaus, R. P. Mason, M. 9 Andersson, and E. M. Sunderland (2012), Multi-decadal decline of mercury in the North 10 Atlantic atmosphere explained by changing subsurface seawater concentrations, Geophys. 11 Res. Lett., 39, L21810, doi:10.1029/2012GL053736. 12 Streets, D. G., Devane, M. K., Lu, Z., Bond, T. C., Sunderland, E. M., Jacob, D. J. 13 (2011), All-time releases of mercury to the atmosphere from human activities, Environ. 14 Sci. Technol., 45, 10485-91. 15 Swain, E. B., D. R. Engstrom, M. E. Brigham, T. A. Henning, and P. L. Brezonik (1992), 16 Increasing rates of atmospheric mercury deposition in midcontinental North America, 17 Science, 257, 784-787. 18 Wang, X., H. Yang, P. Gong, X. Zhao, G. Wu, S. Turner, and T. Yao (2010), One 19 century sedimentary records of polycyclic aromatic hydrocarbons, mercury and trace 20 elements in the Qinghai Lake, Tibetan Plateau, Environ. Pollut., 158, 3065-3070. 11 1 Wu, Y. H., X. Jiang, E. Liu, S. Yao, Y. Zhu, and Z. Sun (2008), The enrichment 2 characteristics of mercury in the sediments of Dongjiu and Xijiu, Taihu lake catchment, 3 in the past century, Sci. China D, 51, 848-854. 4 Yang, H. D., R. W. Battarbee, S. D. Turner, N. L. Rose, R. G. Derwent, G. Wu, and R. 5 Yang (2010), Historical reconstruction of mercury pollution across the Tibetan Plateau 6 using lake sediments, Environ. Sci. Technol., 44, 2918-2924. 7 Yang, H. D., D. R. Engstrom, and N. L. Rose (2010), Recent Changes in Atmospheric 8 Mercury Deposition Recorded in the Sediments of Remote Equatorial Lakes in the 9 Rwenzori Mountains, Uganda, Environ. Sci. Technol., 44, 6570-6575. 10 Young, D. R., J. N. Johnson, A. Soutar, and J. D. Isaacs (1973), Mercury concentrations 11 in dated varied marine sediments collected off Southern California, Nature, 244, 273- 12 275. 13 Zhang, Y., L. Jaeglé, A. van Donkelaar, R. V. Martin, C. D. Holmes, H. M. Amos, Q. 14 Wang, R. Talbot, R. Artz, S. Brooks, W. Luke, T. M. Holsen, D. Felton, E. K. Miller, K. 15 D. Perry, D. Schmeltz, A. Steffen, R. Tordon, P. Weiss-Penzias, and R. Zsolway (2012), 16 Nested-grid simulation of mercury over North America, Atmos. Chem. Phys., 12, 6095- 17 6111. 12