Rabies Vaccination Outpatient Orders
advertisement

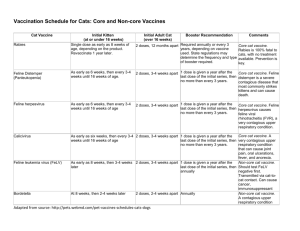
Insert Your Institution Name and Contact Information Here Vaccine Information: Rabies ABOUT THE DISEASE Rabies is a serious disease caused by a virus that enters the body when a person is bitten by an infected animal or when the animal’s saliva comes into contact with an open wound or mucous membranes (eyes, nose, or mouth). The result is a brain infection (encephalitis). Left untreated, rabies nearly always leads to death. Symptoms can appear anytime between 10 days to over 1 year after the bite. Symptoms start as a flu-like illness and progress to throat spasms, seizures, hallucinations, weakness, coma, and death. ABOUT THE VACCINE Getting vaccinated is the best way to protect against rabies after exposure to an infected animal or after exposure to an animal that cannot be captured when rabies has been reported among other animals in the area. The vaccine works by building up your own immunity to the virus. There are 4 shots in total. After today, you will need to receive additional shots in 3, 7, and 14 days. When you received your first shot of the vaccine, you likely also received a shot of rabies immune globulin. This gives you immediate protection until your own immunity becomes active. If you have received the rabies vaccine in the past, you will get 2 shots of the vaccine: one immediately after the bite, and the second shot on the third day. You will not need the rabies immune globulin. Before getting this vaccine, tell the healthcare provider about any of the following: You had a serious reaction to rabies vaccine in the past You are currently ill with a fever You are taking any medication that lowers the immune system, such as chloroquine, cortisone, or prednisone You have HIV or AIDS or another disease that lowers the immune system You have cancer or are getting cancer treatment Even if you have another illness, you should still get the rabies vaccine if you have been exposed to the rabies virus. Your healthcare provider can answer any questions you have. Most common risks associated with the rabies vaccine Risks of this vaccine include Pain or swelling at the injection site Nausea, abdominal pain, headache, or dizziness Hives, joint pain, fever Weakness, tingling or numbness, vision problems, or paralysis (all rare) **To receive additional doses of the rabies vaccine to complete the series, please contact [insert phone number] or [insert phone number] to schedule follow-up appointments with the [insert department here] at [insert your institution here]. On the day of the follow-up visit, please report to [insert location here] to register. PLEASE REMEMBER TO BRING YOUR RABIES VACCINATION OUTPATIENT ORDERS FROM THE EMERGENCY DEPARTMENT WITH YOU** When you have received your last dose of the vaccine, please contact the [insert county here] County Health Department at [insert phone number]. IF YOU HAVE A SERIOUS REACTION TO THIS VACCINE You can request that your healthcare provider file a Vaccine Adverse Event Report form, or call 800-822-7967 for more information. TO LEARN MORE To find out more about the rabies vaccine and other vaccines, contact the Centers for Disease Control and Prevention (CDC) at 1-800-232-4636 or http://www.cdc.gov/vaccines/. For additional patient education material, go to www.rabieswatch.com. Courtesy of UM Upper Chesapeake Health US/HB/0515/0029 Place patient label here Rabies Vaccination Outpatient Orders (Page 1 of 1) Open box equals Prescriber's option; must check to order. Checked boxes = automatically initiated unless unchecked. Patient Instructions: Call [insert phone number] to schedule your vaccination appointments Date: Time: □ Institute the “Rabies Vaccination Outpatient Protocol” VACCINE ORDER: Administer per Protocol: (Note: Initial dose given in ED or clinic is Day 0 [Dose 1]) □ lmmunocompetent patients (pediatric* and adult dose): one dose of rabies vaccine, 1 mL IM, should be administered on: (select one schedule) □ Day 3 □ Day 4 □ Day 5 and Day 7 (Dose 2 date) and Day 14 (Dose 3 date) and Day 8 (Dose 2 date) (Dose 4 date) and Day 15 (Dose 3 date) and Day 9 (Dose 2 date) (Dose 4 date) and Day 16 (Dose 3 date) (Dose 4 date) □ lmmunocompromised patients (pediatric* and adult dose): one dose of rabies vaccine, 1 mL IM, should be administered on: (select one schedule) □ Day 3 and Day 7 (Dose 2 date) □ Day 4 and Day 14 (Dose 3 date) and Day 8 (Dose 2 date) □ Day 5 and Day 15 (Dose 3 date) and Day 9 (Dose 2 date) and Day 28 (Dose 4 date) (Dose 5 date) and Day 29 (Dose 4 date) and Day 16 (Dose 3 date) (Dose 5 date) and Day 30 (Dose 4 date) (Dose 5 date) □ Previously vaccinated patients (immunocompetent and immunocompromised; pediatric* and adult dose): one dose of rabies vaccine, 1 mL IM, should be administered on: (select one day only) □ Day 3 (date) -or- □ Day 4 (date) -or- □ Day 5 PEDIATRIC ALLERGIC REACTION ORDERS: Pediatric Patient Weight: (date) kg □ Pediatric Patient (any patient less than 18 years): (prescriber MUST provide patient weight in kilograms) Non-Severe Reaction: □ SDS RN to escort patient to ED for evaluation/treatment Severe Reaction: (serious systemic, anaphylactic, or neuroparalytic reaction) □ Call PEDIATRIC RRT □ Epinephrine PEDIATRIC rescue dose: (if not allergic) (Ped, 30-49 kg): epinephrine (EpiPen) 0.3 mg IM times 1 dose; dose may be repeated in 5-15 min using an additional EpiPen if anaphylactic symptoms persist (Ped, 15-29 kg): epinephrine (EpiPen Jr) 0.15 mg IM times 1 dose; dose may be repeated in 5-15 min using an additional EpiPen Jr if anaphylactic symptoms persist (Ped, 14 kg or less): epinephrine 0.01 mg/kg equals mg IM times 1 dose; dose may be repeated in 5-15 min if anaphylactic symptoms persist □ Transport patient to ED for further evaluation and treatment ADULT ALLERGIC REACTION ORDERS: □ Adult Patient (18 years or older): Non-Severe Reaction: □ SDS RN to escort patient to ED for evaluation/treatment Severe Reaction: (serious systemic, anaphylactic, or neuroparalytic reaction) □ Call RRT □ Epinephrine rescue dose: (if not allergic) (Adult): epinephrine (EpiPen) 0.3 mg IM times 1 dose; dose may be repeated in 5-15 min using an additional EpiPen if anaphylactic symptoms persist □ Transport patient to ED for further evaluation and treatment Authorized Prescriber Signature: Form #: XXXXXXXX XX/XXXX Date: Time: (for verbal/telephone orders) *Note: Weight-based mg/kg dosing is not available for rabies vaccine. Courtesy of UM Upper Chesapeake Health US/HB/0515/0029