Supplementary Table 4 | Summary of observational studies that
advertisement
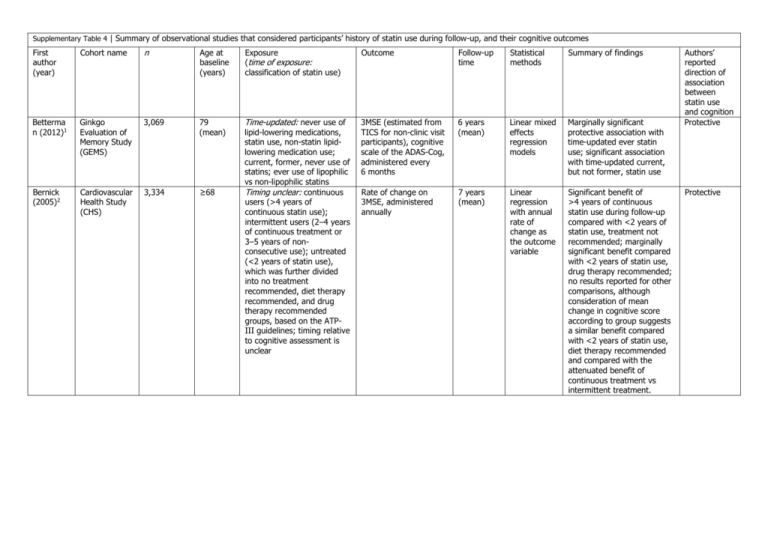
Supplementary Table 4 | Summary of observational studies that considered participants’ history of statin use during follow-up, and their cognitive outcomes First author (year) Cohort name n Age at baseline (years) Exposure (time of exposure: classification of statin use) Outcome Follow-up time Statistical methods Summary of findings Betterma n (2012)1 Ginkgo Evaluation of Memory Study (GEMS) 3,069 79 (mean) Time-updated: never use of 3MSE (estimated from TICS for non-clinic visit participants), cognitive scale of the ADAS-Cog, administered every 6 months 6 years (mean) Linear mixed effects regression models Marginally significant protective association with time-updated ever statin use; significant association with time-updated current, but not former, statin use Bernick (2005)2 Cardiovascular Health Study (CHS) 3,334 Rate of change on 3MSE, administered annually 7 years (mean) Linear regression with annual rate of change as the outcome variable Significant benefit of >4 years of continuous statin use during follow-up compared with <2 years of statin use, treatment not recommended; marginally significant benefit compared with <2 years of statin use, drug therapy recommended; no results reported for other comparisons, although consideration of mean change in cognitive score according to group suggests a similar benefit compared with <2 years of statin use, diet therapy recommended and compared with the attenuated benefit of continuous treatment vs intermittent treatment. ≥68 lipid-lowering medications, statin use, non-statin lipidlowering medication use; current, former, never use of statins; ever use of lipophilic vs non-lipophilic statins Timing unclear: continuous users (>4 years of continuous statin use); intermittent users (2–4 years of continuous treatment or 3–5 years of nonconsecutive use); untreated (<2 years of statin use), which was further divided into no treatment recommended, diet therapy recommended, and drug therapy recommended groups, based on the ATPIII guidelines; timing relative to cognitive assessment is unclear Authors’ reported direction of association between statin use and cognition Protective Protective At the end of follow-up: non- AD dementia (NINCDS– ADRDA, n = 25; n = 20 statin non-users, n = 4 statin users, n = 1 mixed statin users, and n = 0 other lipidlowering medication users) 4 years (maximum ) Cox proportional hazards model Significant reduction in risk of AD dementia with statin use Protective 73 (mean) At the end of follow-up: Change in performance on 10 neuropsychological tests and the CDR-SOB, administered annually 3 years (mean), minimum of 3 annual visits Linear mixed effects regression model Protective 478 11 (mean) At the end of follow-up: Difference in performance on the Moray House Test of intelligence at age 11 years and age 80 years 69 years (mean) 2,004,692* 30–84 At the end of follow-up: Dementia (EMR codes) 7 years (maximum ) General linear modelling using a repeated measures design (reported F-test and proportion of variance explained quantified as a partial η2) Cox proportional hazards model Significant beneficial effect of statin use on change in the CDR-SOB, marginally significant benefit of statin use with respect to change in MMSE scores, no association between statin use and change on the 10 other cognitive tests Significant benefit of selfreported statin use at followup with respect to difference in intelligence scores at age 11 years and age 80 years Null overall, although consideration of individual statins suggested a protective association of simvastatin and atorvastatin use in women Null Sparks (2008)3 Alzheimer's Disease Antiinflammatory Prevention Trial (ADAPT) 2,233 Steenland (2013)4 Uniform Data Set maintained by the National Alzheimer’s Coordinating Center 3,607 Starr (2004)5 Lothian Birth Cohort followup of the 1932 Scottish Mental Health Survey HippisleyCox (2010)6 QResearch ≥70 users of lipid-lowering drugs (never or early and infrequent statin use); statin users (report statin use at all visits, excluding the 1 month visit); non-statin lipidlowering drug users (nonstatin lipid lowering drug at over half of study visits); mixed statin users (intermittent statin users, with at least half of visits on statin therapy) always report statin use versus never report statin use statin use (yes/no) initiation of statin use during follow-up vs never statin use during follow-up Protective Chou (2014)7 Longitudinal Health Insurance Database 2000 16,699 statin users and 16,699 matched non-users of statins 60–100 Beydoun (2011)8 Baltimore Longitudinal Study of Aging (BLSA) 1,604 ≥50 (mean 58) At the end of follow-up: Dementia (ICD-9-CM EMR codes) 5 years (mean), maximum 11 years Cox proportional hazards model Time-updated: never or ever use of statins; Defined at the end of follow-up: never or All-cause dementia (DSM-III-R, n = 259), AD dementia (NINCDS– ADRDA, n = 182) 25 years (median) Not reported initiation of statin use during follow-up vs never statin use during follow-up ever use of statins Significant benefit of statin use during follow-up with respect to incident dementia; stronger associations with higher-potency statins and longer cumulative duration of statins; no difference in association by lipophilicity of statins or age of participants, authors conclude differences in association by sex Ever statin use defined at the end of follow-up was associated with a reduced risk of dementia, AD dementia and MCI. Protective Protective *Prior to exclusion for pre-existing dementia code. Abbreviations: AD, Alzheimer disease; ADS-Cog, Alzheimer's Disease Assessment Scale, cognitive subscale; ATP-III, Third Adult Treatment Panel of the National Cholesterol Education Program; CDR-SOB, Clinical Dementia Rating—Sum of Boxes; DSM-III-R, Diagnostic and Statistical Manual, 3rd Edition, Revised; EMR, electronic medical records; ICD-9-CM, International Classification of Diseases, 9th Revision, Clinical Modification; MCI, mild cognitive impairment; MMSE, Mini-Mental State Examination; 3MSE, Modified Mini-Mental State Examination; NINCDS–ADRDA, National Institute of Neurological and Communicable Disorders and Stroke—Alzheimer's Disease and Related Disorders Association; TICS, Telephone Interview for Cognitive Status. 1. 2. 3. 4. 5. 6. 7. 8. Bettermann, K. et al. Statins, risk of dementia, and cognitive function: secondary analysis of the ginkgo evaluation of memory study. J. Stroke Cerebrovasc. Dis. 21, 436–444 (2012). Bernick, C. et al. Statins and cognitive function in the elderly: the Cardiovascular Health Study. Neurology 65, 1388–1394 (2005). Sparks, D. L. et al. Reduced risk of incident AD with elective statin use in a clinical trial cohort. Curr. Alzheimer Res. 5, 416–421 (2008). Steenland, K., Zhao, L., Goldstein, F. C. & Levey, A. I. Statins and cognitive decline in older adults with normal cognition or mild cognitive impairment. J. Am. Geriatr. Soc. 61, 1449–1455 (2013). Starr, J. M. et al. Life long changes in cognitive ability are associated with prescribed medications in old age. Int. J. Geriatr. Psychiatry 19, 327–332 (2004). Hippisley-Cox, J. & Coupland, C. Unintended effects of statins in men and women in England and Wales: population based cohort study using the QResearch database. BMJ 340, c2197 (2010). Chou, C.-Y., Chou, Y.-C., Chou, Y.-J., Yang, Y.-F. & Huang, N. Statin use and incident dementia: a nationwide cohort study of Taiwan. Int. J. Cardiol. 173, 305–310 (2014). Beydoun, M. A. et al. Statins and serum cholesterol’s associations with incident dementia and mild cognitive impairment. J. Epidemiol. Community Health 65, 949–957 (2011).