molecular genetics
advertisement

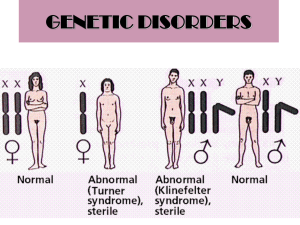
MOLECULAR GENETICS Introduction In recent years, the field of medical genetics has been expanding our knowledge in the involvement of genes and chromosomes with certain diseases and disorders. It is now well established that genetic aspects are responsible for a variety of birth defects, chronic diseases and a high percentage of mental retardation. The Molecular Genetics Laboratory at Biolab provides comprehensive DNA-based diagnostic testing for a variety of genetic conditions and diseases, as well as prenatal diagnosis, presymptomatic, predictive and carrier testing. A new test, the Quantitative Fluorescent Polymerase Chain Reaction (QF-PCR), has been introduced to perform rapid prenatal diagnoses of common chromosome aneuploidies. The clinical utility of this assay has repeatedly been confirmed together with its high sensitivity and specificity in detecting major chromosome abnormalities. One of the advantages of QF-PCR is the automation of part of the procedure that allows high throughput of samples; this makes rapid prenatal diagnosis available to all pregnancies either as a tool to reduce parental anxiety while waiting for completion of fetal karyotype or to improve pregnancy management in case of abnormal result. The Molecular Genetics Laboratory combines state-of-the art genetic testing run by a team of qualified consultants, skilled specialized scientists working in a professional environment dedicated to provide quality diagnostic services constantly aligned with the rapidly growing field of laboratory medicine. Indications for Molecular Genetics Testing Common reasons for referral of molecular genetic tests Thrombosis Screen For the evaluation of inherited thrombotic risk within families. Transient ischemic attack or premature stroke Hyperhomocysteinemia, low plasma folate levels. Prior to pregnancy, oral contraceptive prescription, estrogen therapy or major surgery if there is a family history of thrombosis Recurrent abortion. Single Gene Disorders 1. Recessive Disorders e.g. Cystic Fibrosis, Familial Mediterranean Fever, Hereditary Hemochromatosis, Spinal Muscular Atrophy Carrier testing and risk assessment in families 2. Dominant Disorders e.g. Osteogenesis Imperfecta Confirmation or exclusion of a diagnosis 3. X – Linked Disorders e.g. Duchenne Muscular Dystrophy, Fragile X Syndrome Carrier testing and risk assessment in families Fertility Testing e.g. Y- Chromosome Microdeletions, Cystic Fibrosis Azoospermia Confirmation or exclusion of a diagnosis Carrier testing and risk assessment in families Hemoglobinopathies e.g. alpha-Thalassemia, beta-Thalassemia, Sickle Cell Anemia Carrier testing and risk assessment in families Tissue Typing and Rh Genotyping Organ transplant Maternal-fetal Rh incompatability which can cause Alloimmune Hemolytic Disease in the fetus or newborn. Oncology e.g. Breast Cancer Genes 1 and 2 (BRCA1 and BRCA 2), Philadelphia Chromosome bcr/abl Translocation Carrier testing and risk assessment in families Presymptomatic testing in individuals at risk of late-onset genetic disorder (patient may present positive family history or positive preliminary testing). Prenatal Testing Prenatal diagnosis is available for the following genetic disorders: Cystic Fibrosis α-Thalassemia β-Thalassemia Sickle Cell Anemia Duchenne and Becker Muscular Dystrophies Fragile X Syndrome Spinal Muscular Atrophy AmnioPCR by QF-PCR for aneuploidy screening of chromosomes 13,18, 21, X, and Y AmnioPCR by QF-PCR (QUANTITATIVE FLUORESCENT POLYMERASE CHAIN REACTION) AmnioPCR by QF-PCR is a rapid screening for the detection of aberrant copy numbers of human chromosomes 13, 18, 21, X and Y in small uncultured amniotic fluid samples. This is not equivalent to a full chromosome analysis and is always followed by a full karyotype. AmnioPCR by QF-PCR can be undertaken for rapid sexing on amniotic fluid. Useful when suspecting Turner syndrome, or where there is a risk of sex linked single gene disorders. Limitations of Molecular Genetics Testing Reliable results are dependent on adequate sample collection, transport, storage and processing procedures. Prenatal diagnosis is affected by maternal cell contamination in amniotic fluid and may therefore complicate the interpretation of test results. On rare instances, technical limitations associated with some of the technologies used may be present. The diagnostic efficiency of the test can be impeded by drug interference. Heparin inhibits the PCR reaction, therefore samples collected in heparin tubes will be rejected and a repeat assay requested. Molecular Genetics Tests Genetic Tests Amnio PCR for aneuploidy screening of chromosomes 13, 18, 21, X and Y, QF-PCR Cardio Vascular Disease Risk Factors, 12 Mutations Profile, PCR Cystic Fibrosis CFTR Gene Mutations (36 mutations), Amniotic fluid Cystic Fibrosis CFTR Gene Mutations (36 mutations), Blood Cystic Fibrosis CFTR Gene Mutations (36 mutations), CVS Duchenne and Becker Muscular Dystrophy (DMD,BMD), Genetic Analysis Duchenne and Becker Muscular Dystrophy (DMD,BMD), Genetic Analysis Duchenne and Becker Muscular Dystrophy (DMD,BMD), Genetic Analysis Factor II G20210A Prothrombin Gene Mutation (for CVD and Recurrent Abortion) Factor V G1691A (Leiden) Gene Mutation ( for CVD and Recurrent Abortion) Familial Mediterranean Fever (12 mutations) Hereditary Hemochromatosis HFE Gene Mutations (H63D, S65C, C282Y), PCR HLA ABC, Blood, PCR HLA B27, Blood, PCR HLA B5, Blood, PCR HLA DR, Blood, PCR Human Oncogenes, Breast Cancer genes (BRCA1, BRCA2), Gene Analysis, PCR MTHFR C677T (for CVD and Recurrent Abortion) Spinal Muscular Atrophy (SMA), Genetic Analysis Spinal Muscular Atrophy (SMA), Genetic Analysis Specimen sample Turn Around Time Amniotic fluid 2 – 3 days 120 EDTA whole blood 7 days 60 Amniotic fluid 17 days 190 7 days 90 17 days 190 18 – 20 days 150 30 days 250 30 days 250 4 days 25 4 days 25 4 days 68 4 days 50 7 days 110 4 days 30 4 days 30 4 days 50 3 weeks 350 4 days 25 EDTA whole blood Chorionic Villus Sampling (CVS) EDTA whole blood Amniotic fluid Chorionic Villus Sampling (CVS) EDTA whole blood EDTA whole blood EDTA whole blood EDTA whole blood EDTA whole blood EDTA whole blood EDTA whole blood EDTA whole blood EDTA whole blood EDTA whole blood EDTA whole blood Amniotic fluid Referral Price/JDs 18 – 20 days 150 30 days 250 Spinal Muscular Atrophy (SMA), Genetic Analysis Chorionic Villus Sampling (CVS) Y Chromosome Microdeletions (6 deletions), PCR EDTA whole blood 7 days 60 Amniotic fluid 7 days 170 7 days 170 α-Globin Gene, α-Thalassemia, PCR, Amniotic Fluid α-Globin Gene, α-Thalassemia, PCR, Blood α-Globin Gene, α-Thalassemia, PCR, CVS β-Globin Gene, β-Thalassemia, PCR, Amniotic Fluid β-Globin Gene, β-Thalassemia, PCR, Blood (21 mutations), (21 mutations), (21 mutations), (22 mutations), (22 mutations), β-Globin Gene, β-Thalassemia, (22 mutations), PCR, CVS EDTA whole blood Chorionic Villus Sampling (CVS) 30 days 250 7 days 170 Amniotic fluid 17 days 175 EDTA whole blood 7 days 75 Chorionic Villus Sampling (CVS) 17 days 175 MICROBIAL GENETICS Introduction Emerging infections are attracting greater attention from the public health and medical communities. Pathologists and other physicians are increasingly aware of the importance of the subspecialty of infectious disease pathology as a tool for diagnosis, monitoring therapy and prognosis as well as research of emerging infections. In view of the progress in the science of molecular biology, the applications of diagnostic microbial genetics has taken a major role in the way clinicians manage their patients utilizing an array of technologies such as PCR, RT-PCR, and Gene sequencing. The microbial genetics laboratory provides a list of essential molecular bacteriological and viral tests that can detect, quantify and genotype these pathogenic organisms. Indications for Microbial Genetics Testing Common reasons for referral of Microbial Genetic tests Confirm or exclude a diagnosis of an infectious disease Monitor viral load in established infected individuals and evaluate the efficacy of antiviral treatment Assess reactivation of infection in transplant patients and evaluate potential disease transmission from prospective donors to tissue transplant recipients Diagnosis of sexually transmitted diseases Limitations of Microbial Genetics Testing Reliable results are dependent on adequate sample collection, transport, storage and processing procedures. Heparin inhibits the PCR reaction, therefore samples collected in heparin tubes will be rejected and a repeat assay requested. Any diagnostic test has its limits with regards to sensitivity and detection limit. In rare instances, the diagnostic efficiency of some tests can be impeded by drug interference. Turn Around Time Referral Price/JDs Nasal swabs, nasopharynge al swabs, serum, plasma 4 days 50 Mycobacterium tuberculosis Body fluids 4 days 25 Herpes Simplex Virus I &II DNA Body fluids, swabs 4 days 30 Body fluids, swabs, warts 4 days 70 Chlamydia trachomatis Body fluids, swabs 4 days 35 Neisseria gonorrhea Body fluids, swabs 4 days 25 Specimen sample Genetic Tests Qualitative Assays H1N1 Influenza Virus RNA Human Papilloma Virus DNA Genotyping Quantitative Assays Hepatitis C Virus RNA, Quantitative Serum 4 days 60 Hepatitis C Virus RNA, Genotyping Serum 4 days 80 Hepatitis B Virus DNA, Quantitative Serum 4 days 60 CYTOGENETICS Introduction Cytogenetic techniques allow for the unambiguous identification of each human chromosome and the detection of aneuploidy and many large structural rearrangements that cause physical and / or mental retardation, congenital anomalies related to inherited conditions, abnormal sexual development, and other defects. New techniques allow for increased resolution of chromosome banding patterns, permitting differentiation of a greater number of abnormalities. Fluorescent in situ Hybridization (FISH) technique (using Chromosome- Gene- or Translocation- specific DNA probes) is used in the detection of genetically important trisomies, diagnosis and classification of various neoplasia and hematological malignancies as well as prenatal and preimplantation diagnosis. The Department's activities fall into three main sectors, namely prenatal diagnosis (amniotic fluid, fetal blood, chorionic villus samples, product of conception, fetal biopsies), constitutional cytogenetics (peripheral blood) and onco-hematological cytogenetics (bone marrow, lymph nodes, solid tumors, and pleural effusions). Indications for Cytogenetics Testing CHROMOSOMAL KARYOTYPING Post-Natal Blood Cytogenetics Testing Dysmorphology/multiple congenital abnormalities suggestive of a chromosome abnormality Ambiguous genitalia/indeterminate gender Unexplained learning difficulties/developmental delay Delayed puberty, gynecomastia or inappropriate secondary sexual development Short stature, amenorrhea in females Oligospermia or azoospermia in males Parental karyotyping after two or more unexplained pregnancy loss. Parental karyotyping after pregnancy loss of an unkaryotyped fetus with multiple congenital abnormalities or intrauterine growth restriction (IUGR) Family history (first degree relatives) of a known chromosome abnormality other than simple aneuploidy due to non-disjunction Suspected family history of chromosome abnormality where the karyotype of the affected individual is not known Conditions such as growth failure, microcephaly, neurological abnormalities, immunodeficiency, and an increased incidence of malignancy Postnatal Cytogenetic Testing on Skin Biopsies and Other Tissues Karyotyping of skin biopsy or other types of tissues from a live patient is usually performed to investigate the possibility of tissue-specific chromosomal mosaicism. This is usually undertaken once a karyotyping has been obtained on a blood sample. Prenatal Cytogenetic Testing (Amniotic Fluid, Chorionic Villus Sampling) High risk of carrying fetus with chromosomal abnormality that may have been detected as a result of a screening test (first or second trimester screen). Intracytoplasmic sperm injection (ICSI) or other medical intervention that increases the probability of chromosome abnormalities. Abnormal ultrasonographic findings indicative of chromosome abnormality. History of chromosome abnormality indicative of increased risk for future pregnancies (Chromosome abnormalities in either parent, a previous pregnancy or the family history) Augmented nuchal translucency Cytogenetic Testing of Fetal/Placental Tissue after Pregnancy Loss Any fetus, stillbirth or neonatal death with congenital abnormality suggestive of a chromosome anomaly or with neural tube defect or with intrauterine growth restriction (IUGR). Unexplained stillbirth or spontaneous abortion. Determine recurrence risk for future pregnancy losses. FLUORESCENT IN SITU HYBRIDISATION (FISH) Rapid Neonatal Aneuploidy Screening by FISH Rapid neonatal screening by FISH for trisomies 13, 18 and 21 and for sex. This is not equivalent to a full chromosome analysis and is always followed by a full karyotyping. Cytogenetic Testing of Bone Marrow and Unstimulated Blood Chronic Lymphocytic Leukemia (CLL) Acute Lymphocytic Leukemia (ALL) Acute Myeloid Leukemia (AML) Myelodysplastic Disorder (MSD) Multiple Myeloma (MM) Lymphoma Cytopenia Cytogenetic Testing of Solid Tumors Ewing Sarcoma Neuroblastoma Lymphoma Hepatoblastoma Germ Cell Tumor Rhabdomyosarcoma Synovial Sarcoma Breast Carcinoma Limitations of Cytogenetics Testing Small Subtle Chromosome Abnormalities Cytogenetic analysis relies on G-band quality and resolution. In general, blood samples give the best quality chromosomes and therefore provide the best chance of detecting small subtle chromosome abnormalities. Chromosomes from other tissues (e.g. amniotic fluid, chorionic villus and products of conception) give poorer quality chromosomes; hence the risk of missing a subtle abnormality is increased. It should be noted that on rare occasions a subtle abnormality may be missed at prenatal diagnosis, only to be diagnosed later on a postnatal blood sample. It should also be understood that even a G-band blood karyotype can never exclude extremely subtle chromosome abnormalities that are at the limit of resolution of light microscopy. Detection of Mosaicism It is well established that although mosaicism may be detected by routine karyotyping it can never be 100% excluded. However, if there is an indication of suspected mosaicism, additional cells will be examined to exclude 10% mosaicism at a 95% confidence level. Interpretation of Mosaicism in Prenatal Diagnosis True mosaicism, when detected prenatally, can be difficult to interpret and a further invasive diagnostic test may be required. Mosaic cell lines may be unevenly distributed between the fetus and extra-fetal tissues leading to false positive and false negative results in the most extreme cases. ‘Confined placental mosaicism' (CPM) is observed in approximately 1-2% of CVS samples. Pseudomosaicism can arise as an artifact of culture and is not representative of the fetal karyotype. This is normally present in only one of two or three independently established cultures and can therefore be interpreted accordingly. In most cases, no further invasive testing is required. Maternal Cell Contamination in Prenatal Diagnosis Maternal cell contamination of chorionic villus and amniotic fluid occurs in approximately 1/250 samples and may occasionally complicate the interpretation of results. Normal Variation Each chromosome pair has a specific and identical G-banding pattern in all individuals. However, variation may occur around the centromeric regions and short arms of some chromosomes that is of no clinical significance. These variations are known as ‘polymorphic variants’, ‘polymorphisms’ or ‘normal variants’. In a few cases, it is necessary to distinguish between such variation and a true abnormality. It may therefore be necessary to karyotype the parents or to carry out further tests on a repeat sample. On rare instances, technical limitations associated with some of the technologies used may be present. Cytogenetics Tests Karyotyping Requirements Specimen Quantity Container Maximum Transit Time Turnaround Time Referral Price Peripheral blood 2 – 4 ml Li-Heparin bottle (sterile, vacutainer, plastic) 48 hours 7 days 43 Fetal blood/Cord 0.5 – 1 ml Li-Heparin bottle (sterile, plastic) 48 hours 7 days 43 Bone marrow and unstimulated blood 2 - 5 ml Li-Heparin bottle (sterile, plastic) 24 hours 5 days 55 Amniotic Fluid 15 – 20 ml Universal container or 16 ml conical tube (sterile, plastic) 24 hours 14 days 75 Chorionic Villus Sampling 2 - 25 mg Universal container (sterile, plastic). In sterile media. 24 hours 14 days 80 Products Of Conception Variable Universal container (sterile, plastic). In sterile saline. 24 hours 14 days 80 Fetal tissue/skin 1 cm3 Universal container (sterile, plastic). In sterile saline 48 hours 14 days 80 Fragile X 2 - 4 ml Li-Heparin bottle (sterile, vacutainer, plastic) 48 hours 7 days 60 Chromosomal Breakage (Fanconi's anemia) 2 - 4 ml Li-Heparin bottle (sterile, vacutainer, plastic) 48 hours 7 days 70 FISH Requirements Tests Quantity Container Maximum Transit Time Average Turnaround Time Referral Price Amniotic Fluid, Blood, Chorionic Villus Sampling (CVS) Trisomy 13 [Patau Syndrome] 55 Trisomy 18 [Edward’s Syndrome] 55 Amniotic Fluid in Universal container or conical tube, (sterile, plastic) Trisomy 21 [Down’s Syndrome] Rapid sexing ( X and Y ) Prader Willi 3 ml Digeorge Miller Dieker Li-Heparin bottle / EDTA whole blood (sterile, plastic) 55 55 Within 24 hours 1-2 working days 200 400 CVS in media (sterile, plastic) Trisomy 21, XY 200 95 Trisomy 13, 18 and 21 130 Trisomy 13, 18, 21 and XY 155 Preimplantation Genetic Diagnosis (PGD) PGD Fixated Blastomere Salinated Slide N/A Within 24 hours 50 For each embryo Bone Marrow and Unstimulated Blood BCR/ABL: t(9;22) [CML/ALL] 70 GLI: (12q13) [CLL] 100 100 ATM: (11q22) [CLL] p53: (17p13) [CLL] DLEU: 13q14 [CLL] ETO/AML: t(8;21) [AML/M2] 1 - 2 ml Li-Heparin bottle (sterile, plastic) 100 24 hours 2 - 3 days 100 100 CBFB: inv(16) [AML/M4] 100 PML/RARA: t(15;17) [AML/M3] 100 TEL/AML: t(12;21) [ALL] 100 MLL: 11q23 [ALL/AML] 100 Deleted q5: [MDS]/[AML] 100 Deleted q7: [MDS]/ [AML] 100 LSI/MYC: t(8q24) [Burkitt lymphoma] (screening) 100 MYC/IGH: t(8;14) [Burkitt lymphoma] 100 BCL1/IGH: t(11;14) [Mantle cell lymphoma] 100 IGH/BCL2: t(14;18) [Follicular lymphoma] 100 LSI/ALK: t(2p23) [Anaplastic large cell lymphoma] 100 Solid Tumors 270 HER-2: [Breast carcinoma] EWSR: [Ewing sarcoma] N-MYC: [Neuroblastoma] FKHR: 13q14 [Alveolar rhabdomyosarcoma] SYT: 18q11.2 [Synovial sarcoma] N/A Paraffin embedded block 270 N/A 3 – 5 days 270 270 270