Review for Final Exam SPRING
advertisement

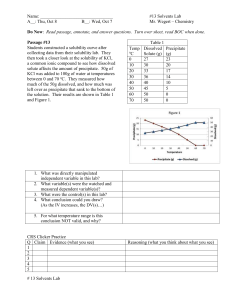
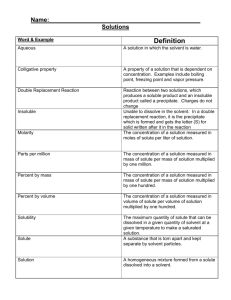
Review for Final Exam SPRING -Concepts Chemistry Name _______________________ Chapter 17 1. What three (3) criteria must be met in order for a reaction to occur? 2. Explain why the criteria identified in question 1 must be met for the reaction to occur? Can you think of an analogy that illustrates this? (for example two train cars need to be coupled together, if the couplers are not in the correct position and there is not enough energy, even though they collide they will not join.) 3. What is activation energy? 4. What is an activated complex? 5. What 3 conditions impact reaction rates and why? 6. What is a catalyst? 7. What happens to the activation energy when a catalyst is present. Explain why. Kinetic-Molecular Theory (KMT), Phase changes, Properties of solids, liquids, and gases (section 10.1, 12.1, 12.2, 12.3) 8. Phase changes (complete the table below-see page 372 for help). How is energy involved? Process known as Solid to liquid 1) Energy is added/removed (circle one) Liquid to gas 2) Energy is added/removed (circle one) Solid to gas 3) Energy is added/removed (circle one) Gas to liquid 4) Energy is added/removed (circle one) Liquid to solid 5) Energy is added/removed (circle one) Gas to solid 6) Energy is added/removed (circle one) 9. The term “kinetic” means motion. So kinetic energy means energy due to particles being in 7) m_______. 10. In all states of matter, there is a competition between the forces of 8)___________________which pull particles together, and 9)__________________ which overcome the attractions and allow particles to move randomly about. 11. The phases of matter depend on the interplay of the competition between those two factors. 12. Use the terms greater than/less than/approx equal to 13. In solids, the kinetic energy will be 10)_________ the effectiveness of the attractive forces. This causes the atoms to be locked in place. But, they 11)v_________ about a fixed point. 14. In liquids, the kinetic energy will be 12)_________ to the effectiveness of the attractive forces. This allows the particles to 13) s______ past each other, but still keeps them close together. 15. In gases, the kinetic energy will be 14)_________ the effectiveness of the attractive forces. This means the particles will be 15)f____ a______ from each other. 16. It is important to note that in solids, liquids, and gases, the strength of the attractive forces does not change—only the effectiveness of them due to how changing the kinetic energy impacts the amount of time particles can interact with each other. 17. How long (approximately) can IMF effectively interact? (indefinitely, long periods, brief periods, never) 18. In solids, intermolecular forces are able to interact 16)________________. 19. In liquids, intermolecular forces are able to interact 17)________________. 20. In gases, intermolecular forces are able to interact 18)________________. 21. When a phase change occurs the mass of the substance 19)________ (will/will not) change. 22. When a phase change occurs the volume of the substance 20)________ (will/will not) change. 23. This means the density of the substance 21)________ (will/will not) change when the phase changes. 24. If particles move closer together, the density 22) ____________ (increases/decreases/stays the same). 25. If particles move farther apart, the density 23)____________(increases/decreases/stays the same). Complete the chart below that compares/contrasts the properties of solids, liquids, and gases Volume Shape Density Compressibility Degree of Diffusion Effectiveness (definite/ (definite/ (High/ (High/ arrangement rates of attractions indefinite) indefinite) med/low) med/low) of (High/ (High/ molecules med/low) med/low) (High/ med/low) Gas Liquids Solids E D C B A 26. If a block of ice is heated, and the temperature is recorded every minute, the result is a temperature-time graph like the one shown above. Note that there are periods of time when the temperature is changing, and there are periods of time when the temperature remains constant. 27. Question A: Identify (using letters) when the temperature is increasing: 24)_____________ 28. Question B: Identify (using letters) when the temperature is remaining constant: 25)_____________ 29. Also note that at all times energy is being added. 30. Based on your answers to Question A, when energy is added, it has the effect of causing the particles to move 26)__________. Based on your answers to Question B, when energy is added it has the effect of causing the particles to overcome the 27)___________________. Boiling 31. In order for a liquid to boil, the 28)___________ pressure from the liquid must be equal to the external pressure (typically atmospheric pressure) around it. Consequently, when this external pressure is changed, the boiling point will also change. Recall the demonstration with the syringe. When warm water (60oC) was placed in the syringe and the plunger was pulled back, the volume increased, causing the pressure within the syringe to decrease. This decrease in pressure makes the 29) e____________ of the attractive forces to be 30)______________ (greater than/less than) they were prior to the pressure change. Meanwhile the kinetic energy has actually 31)___________. The overall effect is that decreasing the external pressure makes it (easier/harder) for the liquid to boil. 32. In a “pressure cooker” as the liquid is heated, the pressure increases, making the effectiveness of the intermolecular forces 32)_____________ (higher/lower). This makes it 33)__________ (easier/harder) to boil. The benefit is that boiling now occurs at a 34)___________ (higher/lower) temperature, making the food to cook 35)___________ (faster/slower). Application 33. Denver has an altitude of about 5430 feet. Minneapolis has an altitude of 841 feet. In which city would you expect water to boil the quickest amount of time?36) ______________ Explain your answer? 37) 34. In which city will food cook the fasted? 38) __________________ Explain your answer? 39) Solutions (sections 13.1, 13.2, 13.3, 14.2, ) Give an example of the following solute solvent mixtures: (see page 396 for help) Gas gas Gas liquid Liquid-liquid Solid-liquid Liquid-gas Liquid-solid Solid gas 35. What is the difference between a solutions, colloids, and suspension? 36. Be able to tell which chemical are soluble and insoluble from the solubility chart. 37. What is an electrolyte? 38. What’s the difference between a strong and weak electrolyte? 39. As temperature of a solvent goes up, what happens to solubility of a gas? 40. As temperature of a solvent goes up, what happens to solubility of a solid? 41. As pressure goes up, what happens to solubility of a gas? 42. What does molarity measure? Solute-solvent interactions 43. The rate at which a solute dissolves in a given amount of solvent can be changed by changing the 71)t_____________ of the solvent, changing the ________ of the particles of the solute, or changing the by 73)s_____________ the mixture to bring fresh solvent into contact with solute. 44. The amount of solute that can be dissolved (the degree of solubility) can be changed by changing the temperature. For many solids, increasing the temperature of a solution will 74)____________(increase/decrease) the amount of solute that can dissolve. 45. Solubility of gases as a function of pressure: 46. If the partial pressure of a gas over a liquid increases, the solubility of the gas 75)_______ 47. If the partial pressure of a gas over a liquid decreases, the solubility of the gas _______ 48. Solubility of gases as a function of temperature: 49. 77) If the temperature of a liquid increases, more/less gas can be dissolved. (circle one) 50. 78) If the temperature of a liquid decreases, more/less gas can be dissolved. (circle one) Application 51. So, to get the most gas to dissolve (i.e, if you wanted to make soda pop super fizzy you would want the temperature to be 79) __________ and the pressure to be _________. 52. To make pop “flat” you would want the temperature to be 81) __________ and the pressure to be _________. Solutions can be unsaturated, saturated, or supersaturated. 53. If a solution is unsaturated, that means 83)__________ (more/no more) solute can be dissolved in the solvent. 54. If a solution is saturated, that means __________ (more/no more) solute can be dissolved in the solvent. 55. An unusual situation can exist known as “supersaturation”. To make this occur the temperature must be increased so that additional solute can be dissolved. Then when the solution is 85)__________, the solute remains dissolved. When something called a “seed crystal” is added to this fragile condition, more of the solute r_______________, coming out of solution. Application 56. Let’s say that sugar has a solubility of 200 grams per 100 grams of water. 57. If you have a solution where 250 grams of sugar has been added to 100 g of water, but not all has dissolved, the solution would be classified as 87)__________________. (sat/unsat/supersat) 58. If you have a solution where 250 grams of sugar has been added to 100 g of water, and all of the sugar has dissolved, the solution would be classified as __________________. (sat/unsat/supersat) 59. If you have a solution where 150 grams of sugar has been dissolved in 100 g of water, the solution would be classified as 89)__________________. (sat/unsat/supersat) 60. If the solubility of a particular solvent is a solution is unsaturated, that means 90)__________ (more/no more) solute can be dissolved in the solvent. Dissociation 61. The term “dissociation” means that when a chemical (solid, liquid, or gas) is placed in water, it 91) b_______ a_______ into i____ with positive and negative charges. When this occurs the solution becomes what is called an ________________ because it conducts electricity. Substances that do not dissociate/conduct electricity are called 94)_____________________. 62. Depending on the makeup of the chemical, the number of ions that form in solution will vary. 63. Application Chemical Dissociates into Total number of ions +1 -1 NaNO3 Na + NO3 2 Ca(NO3)2 Al(NO3)3 Na2S KBr Colligative properties 64. A colligative property is one that does not depend on the type of chemical, but the amount of chemical that is added to a solution, not what chemical is used. (Specifically, the ratio of solute particles to solvent molecules.) There are four colligative properties: freezing point depression (making the f.p. 96)_______ than normal), boiling point elevation (making the b.p. ________ than normal), vapor pressure, and osmotic pressure. Application 65. The normal boiling point of water is 100oC. 66. If 1 mole of “solute A” is dissolved in 1.00 kg of water, the new boiling point will be appox. 100.5 oC. 67. If 1 mole of “solute B” is dissolved in 1.00 kg of water, the new boiling point will be appox. 100.5 oC. 68. If 1 mole of “solute C” is dissolved in 1.00 kg of water, the new boiling point will be appox. 100.5 oC. 69. If 0.5 mole of “solute A” is dissolved in 1.00 kg of water, the new boiling point will be appox. 97)_____. 70. If 2 moles of “solute B” is dissolved in 1.00 kg of water, the new boiling point will be appox. ______. 71. If 3 moles of “solute C” is dissolved in 1.00 kg of water, the new boiling point will be appox. 99)______. 72. Notice, it is not which chemical (A, B, or C) that affects the boiling point. It’s only the a_________ that matters. This same pattern holds for lowering the freezing point, lowering the vapor pressure, and increasing the osmotic pressure. Acid/Base (sections 15.1, 15.2, 16.1 76. List the five properties of acids: 1. 2. 3. 4. 5. 77. List the five properties of bases: 1. 2. 3. 4. 5. 78. Three terms were interchangeable H+_____________ H3O+______________ H+ ____________ 79. Acids have a pH that is __________ Bases have a pH that is ________. Neutral solutions have a pH of _____. 80. What’s an Arrhenius acid? Arrhenius base? 81. Give an example of: Monoprotic acid diprotic acid triprotic acid 82. Give an example of: monobasic dibasic tribasic 83. Why does water have a pH of 7? 84. What is “self-ionization”? 85. Acid + metal yields: 86. Acid + base yields: 87. Acid + carbonate yields: 88. Know how to predict products in a chemical reaction. 89. Be able to identify which (if any) of the products will be precipitates 90. What are the evidences of a chemical reaction? Oxidation-reduction (19.1, 19.3, 19.4) 91. OIL RIG What’s it mean? _____________________________________________________ Cu(NO3)2 LiOH Li2SO4 NaMnO4 K2Cr2O7 92. Write the oxidation numbers for each element: 93. What’s an oxidizing agent do? Na 94. What’s a reducing agent do? Gases (10.1, 10.2, 10.3) 95.) Draw the appropriate graph for the following scenarios: P vs V Who’s law? V vs. n Who’s principle? P vs. 1/V V vs. T (oC) Who’s law? P vs. T (oC) Who’s law? P vs. T (K) V vs. T (K) 96) The process by which a gas spreads throughout a room is called _________________. 97) A “real gas” is most like an ideal gas when the temperature is ____________ and the pressure is _________. 98. What does “STP” stand for? ____________________________________________ 99. To measure the pressure of the atmosphere you use a ___________________. 100. A fixed volume of oxygen is at 25.0oC and has a pressure 101.3 kPa. If the temperature changes to 80oC: Will the pressure be higher, lower, or remain the same? 101. A gas at 700 mm Hg and 0.0oC is cooled to –40oC: Will the pressure be higher, lower, or remain the same? 1 atm = 760 mm Hg = 101.3 kPa = 14.7 psi K = C + 273 P1V1 = P2V2 T1 T2 102. Why must the temperature be converted to Kelvins when doing gas law problems? (Hints: what happens to the pressure if the temp changes from 10oC to 20oC? what if the temp is 0oC? what if the temp is -10oC?) 103. Standard temperature is _______C or ________K. 104. Standard pressure is _____________or ___________ or ____________. 105. What causes atmospheric pressure? 106. How is pressure related to force?______________ to area? ___________ 107. In gases, compared to the kinetic energy, the attractive (van der Wall) forces are ________________strong/weak. 108. In liquids and solids, the van der Wall forces are _________________ compared to the kinetic energy. Show your work for the following calculations and include the appropriate units in your answer: 110. A gas has a volume of 10.0 L at -25oC and 101.3 kPa. What temperature (in oC) is required to cause the gas to have a volume of 17.5 L at 110.0 kPa ? T P V Initial Final Predicted Effect