Syllabus - Holland Public Schools
advertisement
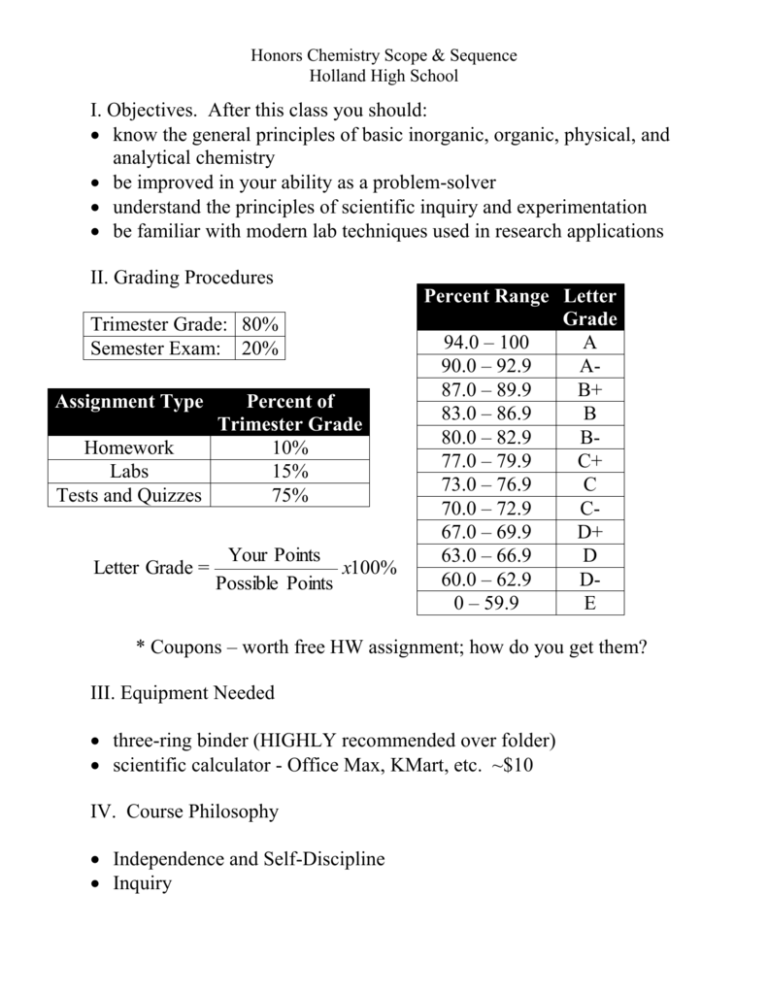
Honors Chemistry Scope & Sequence Holland High School I. Objectives. After this class you should: know the general principles of basic inorganic, organic, physical, and analytical chemistry be improved in your ability as a problem-solver understand the principles of scientific inquiry and experimentation be familiar with modern lab techniques used in research applications II. Grading Procedures Trimester Grade: 80% Semester Exam: 20% Assignment Type Percent of Trimester Grade Homework 10% Labs 15% Tests and Quizzes 75% Letter Grade = Your Points x100% Possible Points Percent Range Letter Grade 94.0 – 100 A 90.0 – 92.9 A87.0 – 89.9 B+ 83.0 – 86.9 B 80.0 – 82.9 B77.0 – 79.9 C+ 73.0 – 76.9 C 70.0 – 72.9 C67.0 – 69.9 D+ 63.0 – 66.9 D 60.0 – 62.9 D0 – 59.9 E * Coupons – worth free HW assignment; how do you get them? III. Equipment Needed three-ring binder (HIGHLY recommended over folder) scientific calculator - Office Max, KMart, etc. ~$10 IV. Course Philosophy Independence and Self-Discipline Inquiry Honors Chemistry Scope & Sequence Holland High School This class is designed to give students a solid foundation in the basic concepts of Chemistry in a manner which should prepare the student to be scientifically literate citizens, as well as to insure that the student is well prepared to take a college level introductory Chemistry course. This class covers all High School objectives described in the Michigan Merit Curriculum for Chemistry (Michigan Department of Education, 2006). The codes for each expectation are included in the heading for each unit below. The document that describes these expectations can be obtained from the Michigan Department of Education (www.mi.gov/mdoe). In addition, our goal is to present the student with a series of relevant laboratory activities and demonstrations which enrich the curriculum and further promote mastery of the concepts Trimester I I. Safety, Laboratory Procedures, and Scientific Measurement (C1.1, C1.2) A. Laboratory Equipment and Laboratory Safety - LAB: Introduction to the Laboratory B. Measurements & Significant Figures C. SI System of Measurement - dimensional analysis D. Scientific Method - LAB: Scientific Method – Experiment Design II. Matter and Energy A. Physical and Chemical Properties and Changes (C5.2) B. Classification of Matter – elements, compounds, and mixtures (P4.p2) C. Density - LAB: Density of Solids and Liquids D. Temperature Units E. Conservation of Matter and Energy (P3.p1, P5.p1) F. Measurement of Heat, conduction (C2.2AB, C3.3A, C5.4A) - LAB: Specific Heat of a Metal G. Kinetic Theory (C2.2f) III. Atomic Theory A. Subatomic Particles, Ions, Isotopes (C4.8ACD, C4.10ABe) B. History of Atomic Theory - important scientists (C4.8Bi) C. Atomic Mass (C4.10cd) - LAB: Atomic Mass of MandMium D. Electromagnetic Spectrum & Behavior of Waves (C2.4x) - LAB: Chemistry in the Dark E. Orbital Diagrams and Electron Configurations (C4.8efh) IV. The Periodic Table A. History and Basic Structure of the Table (C4.9A) - LAB: Periodic Law B. Metals and Nonmetals (C4.9b) C. Valence Electrons (C4.8g) D. Ionization Energy, Electronegativity, Atomic Radii – trends on the Periodic Table (C4.9c) E. Groups and Families on the Table (C4.9A) V. Chemical Nomenclature A. Polyatomic Ions B. Names and Formulas for ionic, molecular, and acidic compounds (C4.2ABcd, C5.5AB) VIII. Chemical Bonding A. Lewis Structures (C5.5c) 1. Covalent Bonding (molecules) - LAB: Building Molecules Honors Chemistry Scope & Sequence Holland High School 2. Ionic Bonding B. Molecular Shapes (VSEPR Theory) and Polarity (C4.4x) C. Intermolecular Forces & Relation to State (C4.3, C4.3x, C5.4ce) 1. Strongest (solid) – Network Covalent, Ionic, Metallic (C2.1c, C5.5de) 2. Middle (liquid) – Dipole-Dipole, Hydrogen Bonding 3. Weakest (gas) – London Dispersion Forces D. Bond Energy & Strength of Bonds (C2.1ab, C3.2x, C3.3x) E. Introduction to Organic Chemistry – nomenclature (C4.2e, C5.8x) IX. Liquids and Solids A. Pressure; 3 phases of matter - Kinetic Theory (C3.3B) B. Phase Changes (P2.p1, P4.p1, C5.4d) - LAB: Heat of Vaporization/Fusion of Water C. Heating and Cooling Curves (C5.4B) - LAB: Endothermic / Exothermic Reactions (CBL) - LAB: Heating and Cooling Curve for Lauric Acid (CBL) D. Phase Diagrams - DEMO DAY: DRY ICE DAY!!!!! X. Gases A. Combined Gas Laws - LAB: P-T Gas Law (CBL) - LAB: Boyle’s Law (CBL) B. Ideal Gas Law (C4.5x) C. Dalton’s Law D. Kinetic Theory of the Gas Laws (C2.2c) Second Trimester VI. Chemical Reactions and Equations A. Balancing Equations (C5.2ABC) B. 5 Types of Reactions 1. Synthesis (C5.7h) 2. Decomposition - LAB: Synthesis/Decomposition Reactions: Magnesium Oxide and Potassium Chlorate 3. Single Replacement (C5.6b) - LAB: Activity Series of the Metals 4. Double Replacement (Precipitation) - LAB: Double Replacement Reactions 5. Combustion - DEMO DAY: COMBUSTION DAY!!!! VII. Stoichiometry A. Formulas 1. Formula Mass; the Mole Concept – Avogadro’s Number (C4.6x, C5.2g) - LAB: Formula Mass of Butane 2. Percent Composition (C4.1a) - LAB: Percent Water in a Hydrate 3. Empirical and Molecular Formulas (C4.1bc) - LAB: Empirical Formula of Silver Oxide B. Equations 1. Mass, mole, molecule conversions (C5.2df) 2. Percent Yield of a Reaction 3. Limiting Reactants (C5.2e) - LAB: Formation of a Precipitate Honors Chemistry Scope & Sequence Holland High School XI. Solutions A. Properties of Solutions (C4.7x) - LAB: Density of Various Sugar Solutions B. Solubility – effect of temperature and pressure (C3.4g) C. Molarity - LAB: Concentration of Solution: Beer’s Law (CBL) D. Preparation of Solutions, dilution - LAB: Solution Making - DEMO DAY: LIQUID NITROGEN DAY!!! XII. Thermochemistry and Kinetics A. Factors That Affect Reaction Rate: temperature, pressure, catalysts, concentration (C2.3a, C5.r1x) - LAB: Iodine Clock Reaction B. Reaction Progression Graph – activation energy (C2.3b, C3.1b, C3.4d) C. Heat of Reaction, Hess’ Law (C3.1acd, C3.4AB) - LAB: Heat of Reaction I D. Entropy and Free Energy (C2.2e, C3.4ef) XIII. Equilibrium A. Equilibrium Expressions (C5.3a) - LAB – Equilibrium Constant for Solubility of Calcium Hydroxide B. LeChatlier’s Principle (C3.4c, C5.3bc) - LAB: The Effect of Concentration and Temperature Changes on a Cobalt Complex Ion XIV. Acids and Bases A. Properties and Definitions of Acids and Bases (C5.7ABCf) - LAB: Acid/Base Indicators B. Lowry-Bronsted Definitions of Acids and Bases (C5.r7i) C. pH, pOH (C5.7Dg) D. Buffer Solutions (C5.7E) - LAB: Properties of Buffer Solutions E. Titrations - LAB: Titration Curve (CBL) - LAB: Concentration of a Weak Acid Solution XV. Oxidation and Reduction A. Oxidation Numbers B. Balancing Redox Equations (C5.6a) C. Galvanic Cells & Electrode Potentials (Eo), cathode, anode (C5.6cde) - LAB: Establishing a Table of Reduction Potentials XVI. Nuclear Chemistry A. Nuclear Transmutations & Nuclear Particles (C2.r5b) B. Nuclear Decay & Half Life (C2.5a) - LAB: Half-Life Simulation C. Fission and Fusion Reactions (C2.r5cd, C3.5a) D. Nuclear Energy – Advantages and Disadvantages











