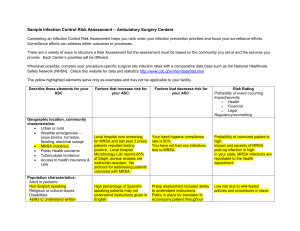
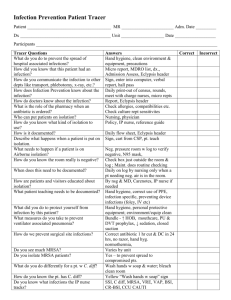
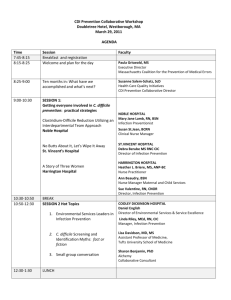
Oral Abstract Presentations - SHEA Spring 2015 Conference
advertisement

May 14, 3:30 – 4:30pm Nutcracker Ballroom 1 Compliance to a four item surgical site infection (SSI) prevention bundle reduces the risk of an SSI Mayke B.G. Koek, MD PhD MSc, Loes C. Soetens, MSc, Titia E.M. Hopmans, BSc, Jan C. Wille, Birgit H.B. van Benthem, PhD and Sabine C. de Greeff, PhD, National Institute for Public Health and the Environment, Bilthoven, Netherlands Background Surgical site infections (SSIs) are a major complication following surgery. In 2008, the Dutch Hospital Patient Safety Program (DHPSP) developed a so-called SSI care bundle, consisting of four interventions aiming at prevention of SSIs: timely antibiotic prophylaxis, normothermia, no hair removal, and hygiene discipline on the OR. The bundle is implemented for selected types of surgery (index surgeries). Reporting of compliance to the bundle is incorporated in the SSI incidence surveillance of the Dutch PREZIES network. This paper describes the reporting of and compliance with the bundle, and investigates the association between incidence of SSIs and adherence to the SSI bundle. Methods PREZIES SSI surveillance data for the index surgeries from 2009 to 2014 were selected. The four interventions were analysed separately as well as combined. Multilevel Log Binomial Regression was used to calculate Relative Risks (RR) on SSIs for bundle compliance, taking into account clustering within specialty and hospitals, and adjusting for the hospitals' participation duration to the DHPSP. Complete compliance to all 4 interventions was compared to incomplete compliance as well as complete noncompliance. Results 199927 surgeries were included. Over the years, reporting of the complete bundle increased from 10% to 57%. Complete bundle adherence among those that were completely reported increased from 9% to 73%. Adherence rates for the 4 interventions separately were higher (figure 1). SSI incidence varied depending on type of surgery (figure 2). Taking into account differences between specialties and hospitals, the RR of SSIs for complete compliance was 0.80 (95%CI 0.67 to 0.95) when compared to incomplete compliance, and 0.82 (0.67 to 1.00) when compared to complete non-compliance. Corrected for duration of the hospitals participation to the DHPSP, these became 0.64 (0.41 to 0.99) and 0.65 (0.40 to 1.06) respectively. Discussion Although compliance increased over time, compliance with the complete bundle can still be improved. We found a positive and significant association between adherence to the bundle and the reduced risk of an SSI. If implementation of the bundle is strengthened, a substantial decrease of SSIs can be achieved for more patients. A Novel Device to Reduce Airborne Colonies and Particulates at Incision Sites During Implantation of Prostheses Rabih O. Darouiche, MD, Baylor College of Medicine, Houston, TX, Sean Self, Nimbic Systems, Stafford, TX and Daniel O'Connor, PhD, University of Houston, Houston, TX Background Airborne microorganisms (CFU) shed on skin squames from operating room personnel contribute to the incidence of prostheses-related infections. The airborne vector remains largely uncontrolled due to factors such as numerous personnel, room traffic, and door openings. Methods to reduce the presence of airborne CFU mitigate environmental factors contributing to such infections. The study objective was to determine if the novel Air Barrier System (ABS) device, which provides localized isolation of incisions, would significantly reduce airborne CFU and particulates at incision sites during prostheses implantations. Methods A single-site, RCT enrolled 300 patients who underwent hip arthroplasty, instrumented posterior spine fusions, and prosthetic vascular graft implantation. Patients were randomly assigned (1:1), to either the experimental group (ABS device) or control group (no device). Airborne CFU and particulates were sampled simultaneously at 10-minute intervals via sterile tubings attached at incisions which drew air onto agar plates and a particle counting device. Particle counts were skewed and underwent Box-Cox transformations. Counts and distributions of CFU (CFU/m3), transformed particulate counts (particles/m3), number of surgical staff, and surgery time were compared between study groups, and associations among CFU, particulate, staff, and surgery time were tested. Generalized linear models with link functions and distributions were used. A multivariate model tested group differences in CFU after adjusting for particulate counts, staff, and surgery time. Robust standard errors were computed to account for clustering of the 10-min intervals within patients. Results There were 291 cases with complete data for all variables, comprising 3587 10-min intervals. Both the CFU and particulate counts were significantly higher in the control group vs. ABS group (Table 1). Number of people present (p=0.792) and surgery time (p=0.645) did not differ between groups. Counts of particulate (p<0.025), staff count (p=0.031), and surgery time (p=0.003) all related to CFU counts. Patients in the ABS group were more likely than in the Control group to have 0 CFUs in a 10-min interval (0 CFU in 70% vs. 49% of intervals, respectively, OR=0.86, p<0.001) and to have a lower average CFU count per interval (2.2 vs. 4.4, respectively, p<0.001) after adjusting for particulate count, staff, and surgery time. Conclusion The results indicate that the ABS can decrease the airborne CFU and particulates at surgical sites. A large, multicenter, RCT is being initiated to assess the impact of this approach on the incidence of infection in patients undergoing placement of prostheses. Skin and Nasal Antiseptic Use in the Prevention of Post-Operative Infections Nicholas A. Flynn, MD, University of Tennessee Nashville Internal Medicine Residency Program, Nashville, TN and Mark Carr, MD, Assistant Professor of Medicine University of Tennessee Nashville, Nashville, TN; Head of Infection Control - St. Thomas Midtown Hospital, Nashville, TN Background Nasal carriers of Staphylococcus aureus are at increased risk of developing infections after surgery. Studies have shown application of skin and nasal antiseptic as directed one hour prior to surgery kills 99.5% of bacteria in the nares, reducing the risk of infection after surgery. Method All patients undergoing surgery were included in this study with the exception of patients allergic to Betadine/iodine. Patients were instructed to apply the 3MTM Skin and Nasal Antiseptic (povodineiodine solution 5% w/w [0.5% available iodine] USP), a preoperative prepping solution designed for use on skin and nasal mucosa tissue, to both nostrils per manufacturer instructions at least one hour prior to surgery. Patients were followed postoperatively and monitored for development of infection. Result From January 2009 to November 2011, 9,135 patients underwent surgical procedures and were monitored for post-operative infection. The experimental intervention began in September 2010 with all surgical patients undergoing pre-treatment with the 3MTM Skin and Nasal Antiseptic. A total of 5,154 surgical patients were followed prior to implementation of the intervention, and 63 (1.22%) of these patients developed post-operative infection. Following initiation of the intervention, 3,981 surgical patients were followed with 18 (0.45%) developing post-operative infection. Statistical analysis sought to determine whether there was an infection rate trend prior to intervention and to compare pre- and post-intervention infection rates. Statistical analysis was performed using Poisson regression with the square root of deviance over the degrees of freedom as the scale parameter. Analysis revealed the following: No trend in infection rate prior to intervention (p-value 0.18) Infection rate for any month pre-intervention was 1.04 times infection rate of the previous month (95% CI, 0.98 to 1.10) Statistically significant decrease in infection rate following experimental intervention (pvalue 0.0029) Conclusion Pre-operative use of 3MTM Skin and Nasal Antiseptic on nasal mucosa prior to surgical intervention resulted in a statistically significant decrease in post-operative infections. May 15, 2015 8:30 - 10:00 am Nutcracker Ballroom 1 Frequent Contamination of the Skin and Clothing of Healthcare Personnel during Removal of Personal Protective Equipment: A Multicenter Evaluation and Educational Intervention Myreen E. Tomas, MD1, Sirisha Kundrapu, MD, MS2, Priyaleela Thota, MD3, Venkata Sunkesula, MD, MS2,3, Jennifer Cadnum, BS2,3, Thriveen Sankar Chittoor Mana, MS2, Annette Jencson, CIC3, Michelle T. Hecker, MD2,4, Amy Ray, MD2,5 and Curtis J. Donskey, MD1,2, (1)Geriatric Research Education and Clinical Centers, Louis Stokes Cleveland VA Medical Center, Cleveland, OH, (2)Case Western Reserve University, Cleveland, OH, (3)Cleveland VA Medical Center, Cleveland, OH, (4)Metrohealth Medical Center, Cleveland, OH, (5)University Hospitals Case Medical Center, Cleveland, OH Background Wearing personal protective equipment (PPE) reduces, but does not eliminate, the risk that healthcare personnel may become contaminated with pathogens. Such contamination may place personnel at risk, as illustrated by recent cases in which healthcare personnel acquired Ebola virus infection. The risk for contamination may be particularly high during PPE removal. However, limited information is available on frequency and routes of personnel contamination during PPE removal. Methods In 4 hospitals, simulations were performed in which healthcare personnel used their usual technique to remove PPE (gloves and gown) that was contaminated with a fluorescent lotion placed either on the gloves or on the front of the gown. PPE removal was observed to assess if correct technique was used and a black light was used to identify sites of contamination on skin and clothing. In one facility, we determined the impact of an educational intervention on frequency of personnel contamination during PPE removal and evaluated the frequency of contamination during removal of full-coverage PPE in Ebola virus training sessions. Results Of 435 PPE removal simulations, contamination of skin or clothing occurred in 200 (46%), with more frequent contamination when incorrect technique was used (Figure). The frequency of incorrect technique did not differ among hospitals (P=0.13) or for different provider types (P=0.26). The hands and neck were the most frequently contaminated sites. The educational intervention resulted in a sustained reduction in skin and clothing contamination during glove and gown removal (73% to 5%; P<0.0001). During 25 assessments of PPE removal in Ebola virus training sessions, contamination of skin or clothing occurred in 2 (8%). Conclusions Contamination of the skin and clothing of personnel occurred frequently during PPE removal, even when no lapses in technique were observed and when only the gown was contaminated. Although less frequent, contamination also occurred during removal of PPE following protocols designed for care of patients with Ebola virus infection. Simple educational interventions can significantly reduce the risk for contamination. Figure 1. Frequency of skin and clothing contamination with during removal of gloves (A) or gowns (B) contaminated with a fluorescent lotion Effect of Contact Precautions on Adverse Events by Patient Report and Chart Review Michael Liquori, MD1, Lindsay D. Croft, MS2, Preeti Mehrotra, MD3, Hannah R. Day, PhD2,4, Elizabeth Lamos, MD1, Ryan Arnold, MD5, Eli Perencevich, MD, MS6, Anthony Harris, MD, MPH2 and Daniel Morgan, MD, MS2,4, (1)University of Maryland Medical Center, Baltimore, MD, (2)Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, (3)Boston Children's Hospital, Boston, MA, (4)VA Maryland Healthcare System, Baltimore, MD, (5)University of Maryland School of Medicine, Baltimore, MD, (6)University of Iowa Carver College of Medicine, Iowa City, IA Background Contact precautions have been reported to decrease healthcare worker visits and increase the number of medical harms. Contact precautions may also lead to increased adverse events. Our objective was to determine the association between contact precautions and adverse events as assessed by patient report and the standardized, electronic Institute for Healthcare Improvement (IHI) trigger tool. Methods A prospective cohort study of inpatients was followed through discharge from January to November 2010 at the University of Maryland Medical Center. Patients were enrolled at admission and frequency matched by hospital unit and length of stay. Patients were evaluated at admission and on hospital day 3, 7, and 14 (until discharged). At each point, patients underwent a standardized interview to identify perceived problems with care. After discharge the standardized interview was administered by telephone. Responses were recorded, transcribed, and coded by two physician reviewers. Additionally, three physician reviewers performed chart reviews to identify adverse events using the standardized IHI trigger tool. Events identified by the IHI trigger tool were then compared to the patient responses and assessed for congruence. Logistic regression analysis was used for our primary analysis of contact precautions and patient adverse events. Results Five hundred twenty eight patients were enrolled, with 296 being frequency matched. Of these 296 frequency matched patients, 84 (28.4%) had adverse events based on chart review. Only 12 of these adverse events were also reported by patients. Adverse events identified by chart review were not associated with contact precautions (OR 1.30; 95% CI 0.76-2.21; p=0.33) after adjusting for age greater than 51 years (OR 1.95; 95% CI 1.13-3.35), length of stay greater than 4 days (OR 2.57; 95% CI 1.054.41), and Charlson comorbidity score (OR 1.04; 95% CI 0.90-1.19). Adverse events reported by patients were rare and also not associated with contact precautions (OR 3.12; p=0.14). Conclusions Patients rarely reported adverse events. Patients on contact precautions were no more likely to experience adverse events than patients not on contact precautions, regardless of method used to identify adverse events. Reinterpreting Compliance with Transmission-Based Precautions: A Sociological Approach Julia E. Szymczak, PhD, The Children's Hospital of Philadelphia, Philadelphia, PA and Susan E. Coffin, MD, MPH, Perelman School of Medicine at the University of Pennylvania and the Children's Hospital of Philadelphia, Philadelphia, PA Background Achieving high levels of healthcare worker (HCW) compliance with transmission-based precautions to prevent the spread of infectious diseases within healthcare facilities remains a challenge. Even after improving HCW knowledge and providing resources, studies demonstrate that compliance does not reach acceptably high levels. The objective of this study was to examine HCW perceptions of the barriers to achieving high levels of compliance with transmission-based precautions. Method We conducted 103 in-depth, semistructured interviews with physicians, nurses and respiratory therapists working at a large academic pediatric hospital. Data were systematically analyzed from a sociological perspective. Themes were identified using a modified grounded theory approach and their frequency calculated. Result The majority of respondents (95, 92.2%) perceived that competing priorities were the primary barrier to achieving high levels of compliance with transmission-based precautions. They explained how tension often arose in their everyday clinical work between the priority to prevent transmission of infectious diseases and other equally, or even more, pressing concerns. Further analysis revealed 3 types of competing priorities that threaten compliance: technical, social and organizational. Technical competing priorities, mentioned by 72 (69.9%) of respondents, are times when transmission-based precautions come in direct conflict with other therapeutic goals or techniques, such as getting to a patient who is rapidly decompensating. Social competing priorities, mentioned by 65 (63.1%) of respondents, are times when transmission-based precautions come in conflict with the social dynamics of interaction with patients, families and colleagues. For example, worry about provoking fear in young children who are placed on droplet precautions. Organizational completing priorities, mentioned by 61 (59.2%) of respondents, are times when HCWs perceive that the organization’s emphasis on cost reduction and increasing clinical productivity come in conflict with transmission-based precautions, such as when two patients are placed in a single bed room. Conclusion The challenge of achieving reliably high levels of compliance with transmission-based precautions is more complex than simply a problem of knowledge or resources. This study elaborates the social dynamics that contribute to noncompliance and suggests that novel interventions are needed to address this problem. In the Face of Emerging Diseases: Using Data to Guide Staff Protection Amber H Mitchell, DrPH, MPH, CPH, International Safety Center, Apopka, FL and Ginger B Parker, MBA, International Safety Center, Charlottesville, VA Background While there has been extensive research on occupational contaminated sharps incidents in healthcare settings, little research has focused on splashes and splatters of blood and body fluids. This is especially important in today’s world where the globalization of travel means that previously geographically isolated infectious microorganisms like Ebola Virus become a real threat to clinicians around the world. We learned that occupational exposure to blood and body fluids deserves renewed focus and post-September 11 public health preparedness deserves restoration. Currently there is not a large evidence base to identify the risks associated with splashes and splatters of blood and body fluids to mucous membranes (e.g. eyes, nose, and face). Over the last ten years, there has been swelling activity in Federal, state, and local policy regarding healthcare-associated infections including not just bloodborne pathogens, but vector- and contact-transmitted pathogens (e.g. MRSA, c. difficile). There are few focused studies that evaluate and measure the occupational impact that splashes and splatters have on workers. Enumeration of risk is more important today than ever given revitalized focus on body fluid exposure, PPE use, and risk of disease. Method This study summarizes quantitative occupational incident/exposure data from 27 aggregate hospitals throughout the U.S. that contribute to the Exposure Prevention Information Network (EPINet™). Incident data includes reports of blood and body fluid exposures across job category, exposure type, location, PPE worn, and procedure type. Result In the calendar year 2012, there were 175 blood and body fluid exposures reported using EPINet in a 27hospital surveillance aggregate. The majority of incidents were blood or visibly bloody urine (71.4%, 72.4% respectively) to the face/head (76.7%). Of those exposures, 60% were to the eyes with only 5.7% of employees wearing goggles as a form of PPE; and 26.9% were to intact skin with only 15.5% wearing a gown or coat as protective apparel. The majority of the reported exposures occurred in the patient’s room, operating room, or emergency department (33.7%, 20%, and 18.3% respectively). Aside from acknowleding that they were not wearing PPE, approximately 25% of workers reporting the incident, indicated that an engineering control, administrative or work practice could have prevented the exposure/incident. Of those, 72.4% cited some form of eye protection would have prevented the exposure. Conclusion The routine, standardized capture of quantitative occupational exposure and incident data allows us to identify not only risk, but modalities or controls for prevention or protection of staff. When emerging infectious diseases like Ebola are now a global reality, capturing this type of data can mean the difference between reacting to possible or theoretical risk to proactively preventing it. Comparison of Hand Hygiene Monitoring Methodologies in Clinical Application: ‘My 5 Moments' versus ‘Entry/Exit' Nai-Chung Nelson Chang, MS1, Andrew Jesson2, Heather Schacht Reisinger, PhD3, Marin Schweizer, PhD2,4, Margaret Graham, MS5, Daniel Morgan, MD, MS6,7, Lisa Pineles, MA8, Graeme Forrest, MD9,10 and Eli Perencevich, MD, MS2, (1)University of Iowa College of Public Health, Iowa City, IA, (2)University of Iowa Carver College of Medicine, Iowa City, IA, (3)CADRE - Iowa City VAHCS and Carver College of Medicine, University of Iowa, Iowa City, IA, (4)Iowa City VA Healthcare System, University of Iowa, Iowa City, IA, (5)Iowa City VA, Iowa City, IA, (6)VA Maryland Healthcare System, Baltimore, MD, (7)Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, (8)University of Maryland, Baltimore, MD, (9)Portland VA Med Center, Portland, OR, (10)Medicine, OHSU, Portland, OR Objectives Directly observed hand hygiene monitoring guidelines recommend monitoring opportunities on “Entry/Exit” or using the WHO “My 5 Moments”. However, few studies have compared the efficiency of each method in capturing hand hygiene opportunities. We aimed to compare the efficiency of each method and whether it varies based on level of care: intensive care unit (ICU) vs non-ICU. Methods Healthcare worker hand-hygiene compliance data was collected by covert observers from June 4 to November 13, 2013 in Baltimore, Iowa City and Portland Veterans Affairs Medical Centers. Observations were conducted in both the surgical/medical wards as well as the ICU in each facility. Data were collected concurrently using both standards methods: “Entry/Exit” and “My 5 Moments”. Entry/Exit” was defined as hand hygiene upon entry or exit of a patient room and the “My 5 Moments” were defined as hand hygiene prior to aseptic tasks and patient contact, and after fluid exposure, patient contact and touching patient surroundings. Each instance of observation was limited to the first hand hygiene opportunity for each of the seven time points in patient care as listed above. Analysis were completed using the z-test and the chi-square test. Results 2,733 observations were included, during which 2,104 “Entry/Exit” and 1,513 “My 5 Moments” opportunities were recorded. Observation rates for the “Entry/Exit” method (76.98%, 95%CI 75.40%78.56%) was shown to be significantly higher than “My 5 Moments” (55.36%, 95%CI 53.50%-57.22%) with p<.0001. The likelihood of observing any “Entry/Exit” opportunities in medical/surgical wards versus ICUs was similar, OR 0.900 (95%CI 0.74-1.08, p=0.26). For “My 5 Moments”, the odds for observing any hand hygiene opportunities were much lower in medical/surgical wards compared to ICUs, OR 0.62 (95%CI 0.53-0.73, p<0.0001). Conclusions While “My 5 Moments” is considered the gold standard for hand hygiene monitoring, “Entry/Exit” appears to be a more viable methodology in the clinical setting, especially when monitoring non-ICU wards. When selecting between Entry/Exit and My 5 Moments, facilities should consider the efficiency of each method and might favor one method over the other based on clinical setting, such as ICU vs non-ICU. Self-reported Hand Hygiene Practices, and Feasibility and Acceptability of Alcohol-based Hand Rubs among Village Healthcare Workers in Inner Mongolia, China Yuan Li, PhD1, Yali Wang2, Daiqin Yan2 and Carol Rao, ScD1, (1)Global Disease Detection Program, United States Centers for Disease Control and Prevention, Beijing, China, (2)Bayan Nur Infectious Disease Hospital, Bayan Nur, China Background Healthcare-associated infections result in substantial morbidity and mortality worldwide. Good hand hygiene, including use of alcohol-based hand rubs (ABHR) and hand-washing with soap and water, is critical to reduce the risk of spreading infections. Limited data are available on hand hygiene practices from rural healthcare systems in China. We assessed the feasibility and acceptability of sanitizing hands with alcohol-based hand rubs (ABHR) among Chinese village healthcare workers and their hand hygiene practice. Methods Five hundred bottles of ABHR were given to village healthcare workers who participated in a public health program in Inner Mongolia Autonomous Region, China. After about one year, standardized questionnaire surveys were conducted among the public health program participants to collect information on their work load, availability and usage of hand hygiene facilities, and knowledge, attitude and practice of hand hygiene. Results Three hundred and sixty-nine (64.2%) participants completed the questionnaire. Although 84.5% of the ABHR recipients believed that receiving the ABHR improved their hand hygiene practice, 78.8% of recipients would pay no more than $1.5 USD out of their own pocket (actual cost $4 USD). The majority (76.8%) who provided medical care at patients’ homes never carried hand rubs with them outside their clinics. In general, self-reported hand hygiene compliance was suboptimal, and the lowest compliance was “before touching a patient”. Reported top three complaints with using ABHR were skin irritation, splashing, and unpleasant residual. Village doctors with less experience or who smoked knew and practiced less hand hygiene. Conclusions This study indicates the need of ABHR and hand hygiene training to improve hand hygiene among village healthcare workers. The overall acceptance of ABHR among the village healthcare workers is high as long as it is provided to them for free/low-cost, but their overall hand hygiene practice was suboptimal. Hand hygiene education and training is needed in settings outside of traditional healthcare facilities. May 15, 2015 10:30 - 12:00 pm Nutcracker Ballroom 1 Epidemiology of Community-Onset and Hospital-Onset Multi-Drug Resistant Escherichia coli Bacteremia: A Nationwide Cohort Study at Veterans Health Administration System Michihiko Goto, MD, MSCI1,2, Jennifer McDanel, PhD1,3, Makoto Jones, MD, MS4,5, Bruce Alexander, PharmD1, Carrie Franciscus, MA1, Kelly K Richardson, PhD1 and Eli Perencevich, MD, MS2,6, (1)Iowa City VA Medical Center, Iowa City, IA, (2)University of Iowa Carver College of Medicine, Iowa City, IA, (3)601 Highway 6 West 152, University of Iowa, Iowa City, IA, (4)Salt Lake City VA Medical Center, Salt Lake City, UT, (5)University of Utah, Salt Lake City, UT, (6)Iowa City VA Medical Center, Iowa City VA Medical Center, Iowa City, IA Background Emerging resistance in Escherichia coli is a great concern. We report the nationwide incidence rate trends and proportion of multi-drug resistant (MDR) isolates in E. colibacteremia over a 9-year period using Veterans Health Administration (VHA) system data. Methods This retrospective cohort includes all E. coli blood culture isolates from patients admitted to acute care beds of 115 VHA hospitals from 46 states between 2003-2012. If the same patient had multiple isolates during one admission, then only first isolate was included. Isolates were classified as community-onset (CO: <48 hours after the admission) or hospital-onset (HO: >=48 hours). Antimicrobial susceptibility results were extracted from electronic medical records system of VHA. Isolates were categorized as non-susceptible, if not susceptible to at least one agent in a specific antimicrobial class. We defined MDR as reported resistance to at least three different classes of antimicrobials, according to CDC/ECDC definition. Chronological trends were assessed by Cochran-Armitage trend test. Results There were 18,212 E. coli blood isolates (CO: 15,475; HO: 2,737) included in the analysis. Of these 5,819 isolates (32.0%) were MDR with HO isolates more likely to be MDR [CO: 4,613 (29.8%) as compared to; HO: 1,206 (44.1%); p<0.0001]. Incidence rates of CO E. coli bacteremia increased from 2.9 per 10,000 outpatients in 2003 to 3.6 in 2012, while HO bacteremia incidence rates were stable. Incidence rates of CO MDR E. coli bacteremia showed steady increase over the study period, while HO MDR bacteremia incidence had a sharp increase in the first half, followed by a slow decline. The proportion of MDR isolates increased among both of CO (27.8% in 2003-2007; 31.4% in 2008-2012; p <0.001) and HO isolates (42.7% in 2003-2007; 45.5% in 2008-2012; p<0.01). Conclusion Within VHA system, we observed steady increases in incidence rates and proportion of MDR among CO E. coli bacteremia isolates. Incidence rates of HO E. colibacteremia had been relatively stable, although there was slow trend towards increasing proportion of MDR isolates among HO isolates. Further studies are needed to analyze risk factors of MDR E. coli bacteremia, in order to guide proper empiric therapy for this serious infection. Prevalence of Invasive Gram-negative Infections due to Carbapenem-resistant Enterobacteriaceae (CRE) Among Adult Patients in US Intensive Care Units (ICUs) Thomas Lodise, MD1, Michael Ye, MS2, Shailja Dixit, MS, MPH, MBA, MD2 and Qi Zhao, M.D. MPH3, (1)Albany College of Pharmacy, New York, NY, (2)Forest Research Inc, Jersey City, NJ, (3)Forest Research Institute Inc., Jersey City, NJ Background CRE is an emerging public health concern but there are scant data on CRE prevalence in ICUs across the USA. This study assessed prevalence of CRE in ICU vs. non-ICU settings across various US geographic regions, using data from a large US hospital database. Methods Retrospective analysis of the Premier hospital inpatient database. Study period: 7/1/2010-12/31/2013. Inclusion criteria: 1) age ≥ 18 y; 2) primary or secondary ICD-9 diagnosis at discharge for a complicated urinary tract infection (cUTI), complicated intra-abdominal infection (cIAI), hospital-associated pneumonia (HAP), or bloodstream infection (BSI); 3) positive culture for Enterobacteriaceae drawn from a site consistent with the infection type; and 4) receipt of antibiotic treatment on day of index culture or ≤ 3 days after index culture. Carbapenem resistance was defined as non-susceptibility to meropenem, imipenem, doripenem, or ertapenem. CRE was also stratified by region, infection type and pathogen. Results 30,875 patients met study criteria. CRE prevalence data are shown in the table and charts. CRE was generally higher in ICU vs. non-ICU settings; the difference was most notable in Mid-Atlantic and Mountain regions (Charts). The highest rates of CRE were noted for Klebsiella spp and Enterobacter spp (Table). Conclusion Overall CRE ranged from 1% to 9% and was higher in ICUs than non-ICUs. The findings highlight the importance of developing decision support systems to identify patients at high risk for CRE, especially in ICU settings. This is critically important for clinicians as culture results are typically not available within the first 3 days. Pathogen Citrobacter E. coli cIAI, cUTI, HAP, BSI Enterobacter Klebsiella Serratia Citrobacter E. coli cIAI Enterobacter Klebsiella Serratia Citrobacter E. coli cUTI Enterobacter Klebsiella Serratia Setting ICU Non-CU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU ICU Non-ICU Patients (n) 550 589 6,217 12,748 1,854 1,386 4,574 4,318 973 645 46 19 494 254 113 36 234 75 19 4 133 411 2,036 8,809 231 629 877 2,249 76 184 % CRE 2.6 1.2 0.9 0.4 7.7 5.0 7.3 5.6 4.1 4.7 2.2 0.0 1.8 0.4 13.3 8.3 6.8 0.0 0.0 0.0 3.8 1.5 0.7 0.3 5.6 5.3 8.3 5.9 5.3 2.2 Variation in carbapenem-resistant Enterobacteriaceae (CRE) active surveillance and inter-facility communication among Chicago hospitals Michael Y. Lin, MD, MPH1, Anne C. Bendelow, BS2, Rosie D. Lyles, MD, MSc2, Karen Lolans, BS1, Mary K. Hayden, MD1, Robert A. Weinstein, MD1,2 and William E. Trick, MD1,2, (1)Rush University Medical Center, Chicago, IL, (2)Cook County Health and Hospitals System, Chicago, IL Background The CDC's Detect and Protect strategy encourages hospitals to be aware of CRE-positive patients; strategies include active surveillance and optimizing inter-facility communication for transfers. CRE detection approaches may vary among short-term & long term acute care hospitals (STACHs & LTACHs). Since Nov 2013, Illinois hospitals can query a patient's reported CRE status in a public health database / information exchange, XDRO registry (xdro.org). Method All STACHs with ≥10 ICU beds in Chicago and LTACHs in Cook County (Chicagoland) were recruited Jan – July 2014; each facility designated 1 infection preventionist to answer a 15-item written questionnaire. We used exact tests for statistical significance. Result 21 of 24 STACHs and 7 of 7 LTACHs responded. 86% of STACHs and 100% of LTACHs reported ≥1 CRE admission/month. CRE admission active surveillance was performed by 2 of 21 (10%) STACHs (high-risk transfers only) and 5 of 7 (71%) LTACHs (all admits), P=0.004. 38% of STACHs reported receiving CRE information on CRE-positive patients “half the time or more” at transfer versus 71% of LTACHs, P=0.20 (Figure). If communication did occur at patient transfer, STACHs were notified via medical record (45%), phone call from the transferring infection preventionist (32%) or care provider (9%), or standardized paper transfer form (27%); LTACHs were notified mostly via medical record and/or standardized transfer form (71% each). 86% of STACHs and 100% of LTACHs reported access to the XDRO registry; 55% of STACHs and 43% of LTACHs queried at least once. Most STACHs did not routinely query (59%) or queried occasionally (32%); none queried every patient. In contrast, 2 of 7 (29%) LTACHs queried all patients at the time of admission. 96% of hospitals indicated interest in automated CRE querying. Conclusion We found heterogeneity in, and a need to improve, CRE detection activity among hospitals. Among STACHs, CRE active surveillance was uncommon and CRE communication from transferring facilities was infrequent. Hospitals, including LTACHs, have begun using the XDRO registry to overcome the shortcomings of traditional inter-facility communication; automation of this process has the potential to improve communication further. Modeling Carbapenem Resistant Enterobacteriaceae (CRE) Control – Rapid Regional Collaboration Produces Greatest Containment Bruce Y. Lee, MD MBA1, Kim F. Wong, PhD2, Sarah M. Bartsch, MPH1, James A. McKinnell, MD3, Loren G. Miller, MD MPH3, Chenghua Cao, MPH4, Alexander J. Kallen, MD, MPH5, Rachel Slayton, PhD, MPH6, Diane Kim, BS4, Shawn T. Brown, PhD7, Nathan Stone, PhD8 and Susan S. Huang, MD MPH4, (1)Public Health Computational and Operation Research (PHICOR), Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, (2)University of Pittsburgh, Pittsburgh, PA, (3)Infectious Disease Clinical Outcomes Research Unit (ID-CORE), Division of Infectious Disease, Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles (UCLA) Medical Center, Torrance, CA, (4)Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, CA, (5)Division of Healthcare Quality Promotion, CDC, Atlanta, GA, (6)CDC, Atlanta, GA, (7)Pittsburgh Supercomputing Center, Pittsburgh, PA, (8)Pittsburgh Supercomputing Center (PSC), Carnegie Mellon University, Pittsburgh, PA Background Carbapenem Resistant Enterobacteriaceae (CRE) are resistant to all but salvage antibiotics. CRE emerged in California in 2010, particularly in long term care facilities. Strategies for containment are uncertain. Methods We used our existing Regional Healthcare Ecosystem Analyst (RHEA) agent-based model of Orange County (OC), CA to simulate the spread of CRE throughout OC's 28 hospitals and 74 nursing homes (NH). The literature and a hospital survey of CRE cases from 2010-2013 helped parameterize CRE prevalence and intra-ward/unit transmission. Our team used RHEA to simulate an intervention (hospital-based rectal screening of all inter-facility transfers and contact precautions for all known cases in both hospitals and NH) under three CRE control scenarios: 1) base case (no intervention), 2) hospitaltriggered response whereby the intervention was implemented when a hospital cumulatively identified 10 known carriers, and 3) a county-triggered regional response whereby the intervention was applied in all facilities once 10 hospitals identified any case of CRE. Results RHEA simulation experiments forecasted that unabated emergence of CRE in 2010 in CA would lead to a 10-year prevalence of CRE carriers (known and undiagnosed) of ~30% in LTACs, ~2.5% in hospitals, and ~10% in NH. Both individualized hospital and county-wide regional screen and isolate strategies markedly reduced the amplification, with regional responses producing a greater and more rapid dampening in both hospitals (including LTACs) and NH (Figure 1). Conclusion Simulation models highlight how intensive inter-facility screening by hospitals and contact precautions by hospitals, LTACs, and nursing homes can blunt amplification of CRE in a region. Coordination of county-wide cooperative strategies is an important public health intervention. A Successful Control Program of a Carbapenem-resistant Enterobacteriaceae (CRE) in a Brazilian Intensive Care Unit (ICU) Claudia Vallone Silva, RN, Hospital Israelita Albert Einstein, São Paulo, Brazil Key words: K. pneumoniae, Carbapenem-resistant Enterobacteriaceae, Infection control. Introduction Carbapenem-resistant Enterobacteriaceae (CRE) is increasingly prevalent pathogens worldwide and has the potential to spread within healthcare facilities. Outbreaks and endemic dissemination of KPCproducing K. pneumoniae pose a challenge to infection control. Objective Report an outbreak of colonization and infection related to CRE in an ICU in a Brazilian hospital and describe the major measures implemented to control and prevent. Methods This study was conducted in a 40-bed medical surgical ICU at Hospital Albert Einstein, Brazil. During culture surveillance aiming at identifying multi resistant microorganisms, an infection control nurse observed an increase of CRE. We investigated clonal relatedness for epidemiological comparison using pulsed-field gel electrophoresis (PFGE). Results In July/August 2014 we identified a significant increase in the number of patients colonized (71%) or infected with CRE carrying gene bla KPC (96.4% K.pneumoniae). The rate ratio demonstrated a significant difference in the following periods 0.65 from Jan-April and 2.47 from May-Aug 2014, which confirmed the outbreak. The PFGE revealed genetic diversity among the 18 CRE isolates, which belonged to nine PFGE types. Five strains of CRE shared the same A PFGE type and six strains of CRE shared the same E PFGE type, suggesting nosocomial transmission (fig.1). During this period, a control team was organized and included infectious disease physicians, infection control nurses and ICU staff. A contact precaution audit was carried out and it was observed non-adherence to: hand hygiene (51%), use of the gown (25%), use of gloves (17%), and in 13% the disinfect product was not available. The team established outbreak control strategies in September 2014 (graph.1). All patients admitted to ICU were tested to identify CRE carrying gene bla KPC to determine the acquisition rate, establish contact precaution of positive patients, raise healthcare workers' awareness on hand hygiene, contact precautions and environmental disinfection. After implementing outbreak control measures we observed a significant decrease rate. Conclusion Cooperation among multidisciplinary team was vital for the design of appropriate infection control measures of CRE. Fig.1 PFGE analysis of genomic DNA from isolates of CRE Graph.1 Carbapenem-resistant Enterobacteriaceae (CRE) carrying gene bla KPC rates at ICU May 15, 2015 3:30 - 4:30pm Nutcracker Ballroom 1 A Novel, Sporicidal Formulation of Ethanol for Glove Disinfection to Prevent Clostridium difficile Hand Contamination during Glove Removal Myreen E. Tomas, MD1, Michelle Nerandzic, BS2, Jennifer Cadnum, BS3, Thriveen Sankar Chittoor Mana, MS3 and Curtis J. Donskey, MD1, (1)Geriatric Research Education and Clinical Centers, Louis Stokes Cleveland VA Medical Center, Cleveland, OH, (2)Louis Stokes Cleveland VA Medical Center, Cleveland, OH, (3)Case Western Reserve University, Cleveland, OH Background Clostridium difficile spores may be acquired on the hands of healthcare personnel during removal of contaminated gloves. Disinfection of gloves prior to removal could therefore be an effective strategy to reduce the risk for hand contamination. We tested the hypothesis that a novel, sporicidal formulation of ethanol would be effective for rapid disinfection of C. difficilespores on gloves. Methods Reduction of toxigenic C. difficile spores inoculated on gloves of volunteers was compared after 30 or 60 second exposures to the sporicidal ethanol formulation, 70% ethanol, and 1:10 or 1:100 dilutions of household bleach; the solutions were applied both as a liquid solution and as a wipe. We also examined the efficacy of the sporicidal ethanol formulation for elimination of spore contamination from the gloves of healthcare personnel interacting with C. difficile infection (CDI) patients or their environment. To determine the potential for the disinfectants to damage clothing, the solutions were applied to pieces of colored cloth. Results In 30 and 60 second liquid applications, the sporicidal ethanol formulation reduced spore levels by 1.4 logs and 2 logs, respectively (P<0.0001); 30 and 60 second wipe application resulted in 2 and >2.5 log reductions, respectively (Figure 1). 70% ethanol applied as a liquid or wipe resulted in a <1 log reduction in spores. Reductions achieved with a 1:100 dilution of bleach were equivalent to the sporicidal ethanol solution, whereas a 1:10 dilution was more effective (>3 log reduction). However, both bleach solutions stained clothing, while the sporicidal ethanol solution did not. The sporicidal ethanol solution was as effective as a 1:100 dilution of bleach for elimination of spore contamination acquired on gloves of healthcare personnel. Conclusions A novel, sporicidal ethanol formulation was effective in rapidly reducing C. difficile spores on gloved hands and did not damage clothing. Further studies are needed to test whether use of the sporicidal ethanol solution will reduce healthcare personnel hand contamination during removal of contaminated gloves. Figure1. Effectiveness of acid alcohol for removal of C. difficile spores from gloves Opting out of Clostridium difficile Infections Antonia L Altomare, DO, MPH1,2, Eileen A. Taylor, BSN1, Peter Solberg, MD1,2 and John Mecchella, DO, MPH1,2, (1)Dartmouth-Hitchcock Medical Center, Lebanon, NH, (2)Geisel School of Medicine at Dartmouth, Hanover, NH Background Clostridium difficile infections (CDI) are a major cause of morbidity and mortality, and while most healthcare-associated infections have been declining, the rate of CDI is on the rise. Hand hygiene and Contact Precautions (CP) are the mainstay for preventing the spread of Clostridium difficile (C. diff) spores. While we regularly track the number of CDI, we have not had an efficient way to track and ensure the use of CP. Method Dartmouth-Hitchcock is a 400 bed rural academic medical center located in Lebanon, NH. A preliminary analysis found that CP was ordered for fewer than 50% of instances when a provider ordered a stool study for C. diff. We developed an opt-out electronic order panel which pre-selects the order for CP at the time the stool study for C. diff is ordered. Our aim was to improve the usage of CP in the setting of suspected CDI. Result We analyzed 1157 inpatient encounters in our pre-intervention period (3/1/12-4/16/13) and 1008 in our post-intervention period (4/17/13-4/1/14). We found an increase in the proportion of patient encounters for which CP was ordered when C. diff testing was ordered from 21% pre-intervention to 96% post-intervention (p<0.001). The proportion of CP orders placed within 2 hours of the stool study order improved from 9% to 95% (p<0.001). Post-intervention, we identified 48 failures (CP order placed ≥ 2 hours after stool study order) by 38 ordering providers who “opted out” of the order panel. While most providers only opted out a single time, 6 providers were repeat offenders, with one provider opting out on 5 different occasions. The majority of failures were resident providers (63%). Conclusion Institution of an electronic opt-out order significantly increased the proportion of patients who had CP ordered in the setting of suspected CDI. While we acknowledge that simply ordering CP does not necessarily reflect its implementation, it is the first step in alerting all care team members of recommended isolation precautions. We expect that with proper hand hygiene and an increased use of CP, the rate of healthcare-associated CDI will decline. Further education will be needed for those providers who continue to opt-out of best practices. Attributable Cost of Clostridium difficile Infection in Hospitalized Pediatric Patients Preeti Mehrotra, MD1, Jisun Jang, MA1, Courtney Gidengil, MD, MPH1,2 and Thomas Sandora, MD, MPH1, (1)Boston Children's Hospital, Boston, MA, (2)RAND, Boston, MA Background Attributable cost of inpatient Clostridium difficile infection (CDI) in adults has been estimated to be $3,000-$15,000 per episode. However, the cost of pediatric CDI has not been determined and may differ because severe disease and poor outcomes are less common in children. We sought to measure the attributable cost of CDI in hospitalized pediatric patients. Methods Using the 2012 Kids’ Inpatient Database (KID) from the Agency for Healthcare Research and Quality (AHRQ), we identified secondary diagnoses of CDI using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnostic code of 008.45. Patients between 2 and 18 years of age who survived until discharge were analyzed. Charges were converted to cost using the KID cost-to-charge ratio. All of the analyses were weighted, clustered and stratified appropriately based on the sampling design of the KID. Propensity scores were calculated based on previously identified predictors of CDI (bacterial and fungal infections; malignancy; solid organ transplant; primary and secondary immunodeficiencies; cystic fibrosis; inflammatory bowel disease; presence of gastrostomy tube). Controlling for propensity score and additional potential predictors for CDI-unrelated cost (surgical procedures; extracorporeal membrane oxygenation; dialysis; central catheter placement; and mechanical ventilation), we performed a regression model with a log transformed cost to estimate attributable cost of CDI. Results We identified 5,484 pediatric hospitalizations (0.34%) associated with CDI. Median unadjusted cost of hospitalization for children with CDI was $19,309.40 (interquartile range (IQR): $8,198.31 - $54,123.22) versus $4,584.92 (IQR: $2,628.38 - $8,769.64) for children without CDI. After controlling for propensity scores and predictors for cost, the mean cost for pediatric hospitalizations associated with CDI was 188.12% greater than those without CDI (P < 0.001). Conclusions Hospitalizations associated with CDI for children had almost triple the cost compared to those without. Given the high economic burden associated with pediatric CDI, it should remain an infection prevention priority. Optimizing National Healthcare Safety Network Laboratory-Identified Event Reporting for Hospital-Onset Clostridium difficile Infection: Does Delayed Diagnosis Make a Difference? Michael J Durkin, MD, Arthur W. Baker, MD, Kristen V Dicks, MD, Luke Chen, MBBS, MPH, Daniel J Sexton, Sarah Lewis, MD, MPH, Deverick Anderson, MD, MPH and Rebekah Moehring, MD, Duke University Medical Center, Durham, NC Background Rates of hospital-onset (HO) Clostridium difficile infection (CDI) are higher using the Laboratory ID (LabID) method compared to traditional surveillance. Delay in sending laboratory tests in patients with community-onset diarrhea may be a modifiable reason for discordance. Methods We performed a prospective observational cohort study of patients admitted to 29 community hospitals over 6 months from the Duke Infection Control Outreach Network. HO CDI cases were identified using both traditional and LabID surveillance definitions to quantify the total number of discordant HO CDI cases. “Delayed diagnosis” LabID HO CDI cases were defined as those with diarrhea onset within 3 days of admission but delay in laboratory testing 4 or more days after admission. We calculated a “corrected” LabID surveillance rate by subtracting the delayed diagnosis cases from the total LabID identified cases and then dividing by 10,000 patient-days (ptd). We calculated and compared incidence rates according to traditional surveillance, LabID surveillance, and “corrected” LabID surveillance for the cohort as a whole and for each hospital. Finally, we ranked individual hospitals by the three calculated rates to determine the effect of delayed diagnosis cases on hospital rankings. Results A total of 425 LabID and 311 traditional HO CDI events were observed over 708,551 ptd. The median LabID HO rate per 10,000 ptd was 5.70 vs. 3.40 for traditional surveillance (p<0.001). There were 126 (26%) discordant HO CDI events between the two surveillance methods; the majority were due to delay in diagnosis (n=106; 85%). Differences between the two surveillance methods were also observed when we ranked hospitals by HO CDI rates. The rate and rank differences dissolved after removal of cases due to delayed diagnosis. For example, median “corrected” LabID HO CDI rate was 3.40 and identical to the median traditional surveillance rate. Similarly, “corrected” LabID HO CDI rates and rankings approximated traditional surveillance rates and rankings for individual hospitals. Conclusion Interventions that reduce delays in diagnostic testing for community-onset diarrhea may lead to LabID HO CDI rates similar to traditional CDI surveillance. May 16, 2015 8:30 - 10:00am Nutcracker Ballroom 1 Lack of Patient Understanding of Hospital Acquired Infection Data on CMS Hospital Compare Max Masnick, BA1, Daniel Morgan, MD, MS1,2, John D. Sorkin, MD, PhD3, Elizabeth Kim, MSPH1, Jessica P. Brown, PhD1, Penny Rheingans, PhD4 and Anthony Harris, MD, MPH1, (1)Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, (2)VA Maryland Healthcare System, Baltimore, MD, (3)Veterans Affairs Maryland Healthcare System Geriatrics Research, Education, and Clinical Center, Baltimore, MD, (4)Department of Computer Science and Electrical Engineering, University of Maryland Baltimore County, Baltimore, MD Background Public reporting of hospital quality data is a key element of healthcare reform in the US. Data for hospital-acquired infections (HAIs) can be especially complex to understand, but no prior studies quantify the public's ability to understand these data. We assessed the interpretability of HAI data as presented on CMS Hospital Compare among patients who might benefit from access to these data. Methods We randomly selected inpatients at a large tertiary referral hospital from June to September 2014. Participants compared hospitals using hypothetical HAI data presented in four different ways, including in the same tabular formats used by CMS. Data included numerators, denominators, SIRS, and interpretations of the SIR for the two hospitals. Accuracy of comparisons was assessed. Results Participants (n=110) correctly identified the better of two hospitals when given written descriptions of the HAI measure 72% of the time (95% CI 66%, 79%). Adding the underlying numerical data to the written descriptions reduced correct responses to 60% (95% CI 55%, 66%). When the written HAI measure description was not informative (identical for both hospitals), 50% answered correctly (95% CI 42%, 58%). When no written HAI measure description was provided and hospitals differed by denominator, 38% answered correctly (95% CI 31%, 45%). Conclusion Current public HAI data presentation methods may be inadequate. When presented with hypothetical numeric HAI data, study participants incorrectly compared hospitals based on HAI data >40% of the time. Further research is needed to identify better ways to convey these important data to the public. Figure 1. Percent correct answers (bars indicate 95% CI) among study participants when comparing hypothetical HAI data for two hospitals. Y-axis labels indicate presentation method for HAI data. "Written descriptions" summarize SIRs. Comparative Risk of Bloodstream Infection among Various Types of Intravascular Catheters Payal K. Patel, MD, Sharon B. Wright, MD, MPH, Linda M. Baldini, RN and Graham M. Snyder, MD, SM, Beth Israel Deaconess Medical Center, Boston, MA Background Few published studies have compared the catheter-associated bloodstream infection (CA-BSI) rate attributable to various catheter types within the same population. The National Healthcare Safety Network surveillance definition for these infections may not capture nuances of those truly attributable to an intravascular catheter due to misattribution or underestimation. In this study, we sought to qualitatively compare the incidence of CA-BSI when considering CA-BSI due to any catheter. Methods In this retrospective observational study, institutional databases were used to analyze all unique patient first intensive care unit (ICU) admissions 4/2009 – 3/2014. The insertion and removal time were recorded for all catheter types except peripheral intravenous catheters (PIV). Catheter duration was counted using status at midnight. CA-BSI were included in the analysis if occurring within 14 days of ICU discharge. Follow-up was censored at ICU discharge or CA-BSI outcome. CA-BSI incidence was calculated serially per 1,000 catheter days for study subpopulations with ≥1 of each specific catheter type, and using CA-BSI due to any catheter type and due to the implicated catheter type. Results 22,803 unique first ICU admissions were analyzed, including 12,953 (56.8%) with ≥1 non-PIV placed during follow-up. 101 CA-BSI occurred due to any catheter type. The incidence of CA-BSI due to any catheter and due to the implicated catheter type among study subpopulations is presented in the table. Additionally, 9 CA-BSI were PIV-associated. CA-BSI per CA-BSI 1,000 catheter-days Catheter Catheter Catheter Catheter Catheter-days specific catheter type present implicated present implicated Central venous catheter 23582 59 31 2.50 1.31 Peripherally-inserted central catheter 12677 38 27 3.00 2.13 Hemodialysis 5393 29 9 5.38 1.67 Port 1390 2 0 1.44 0 Arterial line 32742 74 22 2.26 0.67 Other large-bore procedural catheters 8668 20 3 2.31 0.35 Study subpopulation, by Conclusions Data from this observational study suggest there is possible misattribution, including underestimation, of CA-BSI using the current surveillance definition, particularly for hemodialysis, arterial, and procedural catheters. Descriptive epidemiology of patients with central vascular lines in the outpatient setting at an academic tertiary care center Steven S Spires, MD1, Mickie Miller, LCSW2, Katie Koss, RN, MSN2, Patty W Wright, MD1 and Thomas Talbot III, MD, MPH1, (1)Vanderbilt University School of Medicine, Nashville, TN, (2)Vanderbilt Home Care Services, Inc., Nashville, TN Background An increasing number of medical conditions are treated in outpatient settings, resulting in more vascular devices being used in the outpatient population. Data on central line utilization and central lineassociated bloodstream infections (CLABSIs) in the outpatient setting are limited. In an effort to better understand this population, we performed a retrospective descriptive analysis. Methods We retrospectively collected data on patients discharged from Vanderbilt University Medical Center with a central vascular catheter (CVC) who were admitted into the care of our affiliated home care service agency. All patients who left the hospital with a CVC in place, requiring home health skilled nursing provided by our affiliated home care agency from 7/1/2012 to 9/30/2013 were reviewed. Patients were identified first by surgical codes suggesting a CVC had been implanted or inserted, then by querying the electronic clinical documentation database for annotation indicating skilled nursing for infusion therapy had been performed. We defined outpatient CLABSI utilizing the National Healthcare Safety Network (NHSN) definition of CLABSI with a modified timeframe of 2 calendar days after discharge to identify outpatient. Results Overall, 917 admissions into home care met our initial screening criteria and 177 admissions were excluded after further review (CVC placed at an outside facility or there was no information on the CVC). These 740 admissions represented 654 unique patients. The majority of CVCs, 81%, were peripherally inserted central catheters (PICC). The CVCs were accessed frequently, with 79% having scheduled access of at least once daily and 24% requiring access for 3 or more times a day. In total 167 (26%) patients required an unexpected healthcare encounter due to their CVC, 45 in the emergency department and 122 in an outpatient clinic. There were 20 CLABSIs identified in 18 unique patients. Time to event differed depending on the type of line. Conclusion While allowing earlier discharge, CVCs and outpatient infusion therapy are not without a small but likely underappreciated morbidity. Better surveillance practices are necessary to fully understand the healthcare burden associated with outpatient CVCs. Is There an Association Between Patient Safety Culture Measures and Catheter-associated Infections? Results from Two National Collaboratives Jennifer Meddings, MD, MSc1, Heidi Reichert, MA1, M. Todd Greene, PhD1, Helen McGuirk, MPH1, Nasia Safdar, MD, PhD2,3, Sarah L. Krein, PhD, RN1,4, Russell Olmsted, MPH, CIC5, Sam Watson, MSA, MT (ASCP), CPPS6, Barbara Edson, RN, MBA, MHA7, Mariana Lesher, MS7 and Sanjay Saint, MD, MPH1,4, (1)University of Michigan, Ann Arbor, MI, (2)University of Wisconsin School of Medicine and Public Health, Madison, WI, (3)The William S. Middleton Veterans Affairs Medical Center, Madison, WI, (4)Veteran Affairs Ann Arbor Healthcare System, Ann Arbor, MI, (5)St. Joseph Mercy Health System, Ann Arbor, MI, (6)Michigan Health & Hospital Association, Okemos, MI, (7)Health Research & Educational Trust, Chicago, IL Research objective The Agency for Healthcare Research and Quality has funded national efforts to reduce hospital rates of central-line associated blood stream infection (CLABSI) and catheter-associated urinary tract infection (CAUTI). These efforts combine evidence-based interventions with socio-adaptive approaches to foster a culture of safety. We hypothesized that hospital units with better safety culture scores would have lower infection rates. Study design Prospective cohort study of acute care intensive care units (ICUs) and non-intensive care units (nonICUs) participating in the CLABSI or CAUTI projects. CLABSI and CAUTI data were collected at baseline and quarterly post-implementation using the National Healthcare Safety Network definition of catheterassociated infections per 1000 catheter days. Safety culture was quantified using items from 3 culture instruments: Readiness Assessment (RA), Hospital Survey on Patient Safety Culture (HSOPS) and Team Checkup Tool (TCT). Safety culture data was collected at baseline for all three instruments and again roughly one year later for HSOPS, quarterly for TCT. Methods 17 culture items focusing on leadership, teamwork and clinical care were selected for analysis based upon a priori hypotheses to have a significant association with CLABSI and CAUTI rates in ICUs and nonICUs. Multilevel models were used to predict outcome rates over time as a function of culture scores measured throughout the project. Due to multiple testing, p<0.01 criteria was used. Results 1487 units from 903 hospitals (CLABSI) and 1030 units from 952 hospitals (CAUTI) were considered for analysis. Response rates for the 3 culture instruments ranged from 20-24% for CLABSI and 38-43% for CAUTI. Infection rates declined during the projects (41% decline for CLABSI, 14.5% decline for CAUTI). No significant associations were found between safety culture measures and outcome rates. Conclusions Using 3 instruments of safety culture in 2 national collaboratives for prevention of CLABSI and CAUTI, we found no association between culture measures and device-associated infection rates. However, the validity of these safety culture measures remains unclear when assessed periodically, at the unit level, with low response rates. Reducing central line-associated bloodstream infections through a socioadaptive intervention focused on maintenance practices Christopher Crnich, MD, PhD1,2, Erin Bailey, MS1, Rosa Mak, MS3, Timothy Hess, PhD4, Nasia Safdar, MD, PhD4, Stevens Linda, DNP, RN3, Dawn Berndt, RN, MS3, Lyndsy Huser, MSN, RN3, Elise Arsenault Knudsen, MS, RN3, Wendy Curran, MSN, RN3, Betts Troy, MSN, RN3, Diane Mikelsons, MSN, RN3 and Deborah Soetenga, MS, RN3, (1)University of Wisconsin School of Medicine & Public Health, Madison, WI, (2)William S. Middleton Memorial VA Hospital, Madison, WI, (3)University of Wisconsin Hospital and Clinics, Madison, WI, (4)University of Wisconsin School of Medicine and Public Health, Madison, WI Background Efforts to reduce central-line associated bloodstream infections (CLABSI) have largely focused on improving central line insertional practices in the ICU. However, a majority of CLABSIs now occur outside the ICU, most likely through gaps in maintenance practices. An ongoing quality improvement initiative at our hospital created an opportunity to examine the impact of non-insertional interventions on facility CLABSI rates. Methods Unit-specific and housewide CLABSI rates were tracked monthly and cross-sectional assessments of maintenance practices were performed during the study (01/2011 - 08/2014). A new needleless connector as well as several preventative interventions (including: 1) education; 2) post-event review process; 3) CHG bathing; and 4) a line-maintenance culture change process [LMCC]) were introduced during the study period. Control charts were used to identify shifts in the hospital trended CLABSI rate. Multivariate Poisson regression models using a first-order autoregressive covariance structure were used to assess impact of different interventions on observed CLABSI rates. Results Despite the introduction of a number of preventative strategies, the hospital's trended CLABSI rate remained stable (2.0 per 1,000 device-days) through November 2013. Implementation of the LMCC intervention was associated with improvements in observed maintenance practices and a downward shift in the hospital's trended CLABSI rate (1.2 per 1,000 device-days). In multivariate analyses, there was a statistically significant association between the LMCC intervention and observed CLABSI rates (rate ratio = 0.59, P = 0.005). Based on our model, none of the other interventions implemented during the study period were associated with significant changes in facility CLABSI rates. Conclusions These results demonstrate that a culture change intervention centered on line maintenance practices can reduce hospital CLABSI rates. Our next steps are to compare results of staff interviews and practice observations on high- and low-performing units. These data will be critical for achieving a better understanding of how this socioadaptive intervention achieved its success and to better disseminate our findings to other settings. May 16, 2015 10:30 - 12:00pm Nutcracker Ballroom 1 Middle East Respiratory Syndrome Corona virus infection among Healthcare Workers in a Tertiary Care Center in Saudi Arabia Ahmed Hakawi, MD, King Fahad Medical City, Riyadh, Saudi Arabia Background To describe the socio-demographic and clinical characteristics and outcome of infection due to Middle East Respiratory Syndrome Corona virus among healthcare workers in King Fahad Medical City, Riyadh, Saudi Arabia. Methods & Materials This is a retrospective chart review of healthcare workers infected with MER CoV from April, 2014 to June 2014. The IRB of KFMC approved the study. Throat-swab, nasopharyngeal swab , tracheal-aspirate, or bronchoalveolar-lavage specimens were obtained and were placed in viral transport medium (Vircell) and they were subjected to real-time reverse-transcriptase–polymerase-chain-reaction (RT-PCR) amplification of consensus viral RNA targets (upE and ORF1b) to detect MERS CoV . Results During the outbreak of MER CoV infection that occurred at KFMC between April, 2014 and June 2014, 23 staff members were infected. Seventy per cent of the cases were female nurses with no prior chronic medical conditions. Staff members from critical areas were worst affected (table 1) and 74% had fever at presentation. Interestingly, gastrointestinal and to a lesser degree articular features were prominent among the cases, in addition to respiratory symptoms (table 2). Fifteen (65%) cases got admitted, while the remaining were isolated at home or charity building specifically designated for infected asymptomatic staff or those with mild disease. Five were admitted to the intensive care unit (ICU). Mean length of stay for those admitted into the hospital was 9 days (5-17days), while for those that stayed in the charity building was 10 days (3-19 days). The mean period of infectivity (time period between first positive PCR and first negative PCR was 10 days (6-21 days). Treatment was mainly supportive. Only one staff member died, while 96% recovered. Conclusion Prior reports of MERS CoV infections were among the aged population with chronic medical conditions. However, secondary cases among healthcare workers were less severe. Identification of mild and asymptomatic cases among healthcare workers and implementation of strict infection control measures can decrease the risk of transmission. Ebola Virus Disease, Infection Control and Environmental Safety in a Biocontainment Unit Jay B. Varkey, MD1, Colleen S. Kraft, MD1, Aneesh K. Mehta, MD1, G. Marshall Lyon, MD, MMSc1, Sharon Vanairsdale, MS, APRN, ACNS-BC, NP-C, CEN1, Patricia L. Olinger, RBP2and Bruce S. Ribner, MD, MPH1, (1)Emory University School of Medicine, Atlanta, GA, (2)Emory University, Atlanta, GA Background In 2014, four patients with Ebola Virus Disease (EVD) were treated in the Serious Communicable Diseases Unit (SCDU) at Emory University Hospital. Strict infection control practices were implemented to avoid environmental contamination and prevent nosocomial transmission of EVD to healthcare workers. Methods As part of standard operating protocol while caring for patients with EVD, all high touch surfaces were wiped at least every four hours using commercially available hospital disinfectant wipes. Body fluids that contacted the patient care area were immediately contained and disinfected using a quaternary ammonium compound (MicroChem, Westborough, MA). After patients with EVD were discharged from the hospital, but before terminal decontamination using vaporized hydrogen peroxide, multiple environmental samples were obtained from high-touch surfaces using a dacron-tipped swab moistened with bacteriostatic saline. Environmental swabs were tested for the presence of Ebola virus (EBOV) using RT-PCR (Biofire Defense, Salt Lake City, UT). All health care providers who entered the SCDU, laboratory technologists, and anyone managing the waste stream were required to measure their temperature and complete a symptom questionnaire twice daily. Results All 9 samples obtained on 3 separate dates tested negative for EBOV by RT-PCR. Samples tested included patient’s personal belongings (phone, tablet computer), high-touch areas (call button and bedrails) and the bathroom environment (commode, toilet seat, sink handles, etc). Forty-three direct care providers, laboratory technologists and environmental services personnel underwent twice daily temperature checks and symptom monitoring. No employee developed Ebola virus disease. Conclusions Ebola virus disease can be managed successfully without causing environmental contamination or occupation related infections with appropriate protocols and preparations. Meticulous attention to infection control practices by a highly motivated, trained and competent staff is critical to the safe care of patients with EVD. Vancomycin Consumption and Resistance Trends in Staphylococcus aureus Across the Military Health System Michael E. Sparks, PhD1, Uzo Chukwuma, MPH2, Robert Clifford, PhD1, Emma Schaller2, Paige Waterman, MD3, Charlotte Neumann, BSN2, Michael Julius, PMP1, Mary Hinkle, MD1 and Emil Lesho, DO1, (1)Walter Reed Army Institute of Research, Silver Spring, MD, (2)EpiData Center Department, Navy and Marine Corps Public Health Center, Portsmouth, VA, (3)Global Emerging Infections Surveillance, Armed Forces Health Surveillance Center, Silver Spring, MD Background Staphylococcus aureus is one of the most important community and healthcare associated pathogens. Having a vancomycin minimum inhibitory concentration (MIC) >1.5µg/mL, although still in the susceptible range, is prognostic for mortality in S. aureus bacteremia, regardless of resistance to methicillin or the treatment administered. Objectives We sought to: 1) determine whether an upward trend in incidence of isolates having therapeutically problematic vancomycin MICs was occurring; 2) learn if resistance patterns correlated with vancomycin usage across a large national healthcare system; and 3) generate near-term predictions for problematic isolate rates. Materials and Methods Monthly counts of de-duplicated isolates having a vancomycin MIC = 2µg/mL (problematic) and MIC ≤ 1.5µg/mL (tractable) were tabulated from Jan 2010 through Dec 2014. Rates of problematic isolates per 1,000 isolates tested were analyzed (“pMIC data”). Local regression-based decomposition disaggregated series into season, trend and remainder components, and Mann-Kendall tests checked for upward or downward monotonic trends. An autoregressive integrated moving average (ARIMA) model fit to the pMIC data was used for forecasting. pMIC data was simultaneously compared with the monthly vancomycin prescription rate per 1,000 inpatient encounters during Jan 2010 through Dec 2013 (“usage data”). Spearman correlation measured the association between series. Results and Conclusions An upward trend in the aggregate pMIC data was observed with good statistical support (τ = 0.67; p = 1.54E-8); this was also seen in the series' trend component (τ = 0.63; p ≤ 2.22E-16). The ARIMA-based predictive model suggested problematic isolate rates of 228 and 254 by Dec 2015 and 2016, respectively, exceeding 2014's mean average rate of 205 by 11% and 24% (see Figure 1). A downward trend in the usage data was seen, both in the aggregate series (τ = -0.56; p = 8.77E-5) and its trend component (τ = -0.86; p ≤ 2.22E-16). Figure 2 presents trend lines for the pMIC and usage data, which were negatively correlated (ρ = -0.83; p < 2.2E-16). Vancomycin consumption does not seem to explain vancomycin MIC trends in S. aureus, thereby warranting study of the roles of other antibiotics. Figure 1: Figure 2: Multi-Site MRSA Colonization: Single Strains Predominate but Multiple Strains More Likely with Healthcare-Associated vs. Community-Associated MRSA Michael S. Calderwood, MD, MPH1,2, Christopher A. Desjardins, PhD3, Michael Feldgarden, PhD4, Yonatan Grad, MD PhD2,5, Diane Kim, BS6, Raveena Singh, MS6, Samantha J. Eells, MPH7,8, James A. McKinnell, MD7,9,10, Steven Park, MD PhD6, Ellena Peterson, PhD11, Loren G. Miller, MD MPH7,10 and Susan S. Huang, MD MPH6, (1)Harvard Medical School and Harvard Pilgrim Health Care Institute, Department of Population Medicine, Boston, MA, (2)Brigham and Women's Hospital, Division of Infectious Diseases, Boston, MA, (3)Broad Institute of MIT and Harvard, Cambridge, MA, (4)National Center for Biotechnology Information, National Institutes of Health, Bethesda, MD, (5)Center for Communicable Disease Dynamics, Department of Epidemiology, Harvard School of Public Health, Boston, MA, (6)Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, CA, (7)Infectious Disease Clinical Outcomes Research Unit (ID-CORE), Division of Infectious Disease, Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles (UCLA) Medical Center, Torrance, CA, (8)Department of Epidemiology, UCLA Fielding School of Public Health, Los Angeles, CA, (9)Torrance Memorial Medical Center, Torrance, CA, (10)David Geffen School of Medicine, Los Angeles, CA, (11)Department of Pathology and Laboratory Medicine, University of California, Irvine School of Medicine, Irvine, CA Background MRSA colonization at multiple body sites is associated with a higher risk of MRSA infection. In addition, non-nasal colonization is important in transmission. This study compares the genetic relatedness of MRSA isolates collected concurrently from nasal and non-nasal sites. Methods We analyzed isolates from Project CLEAR, a clinical trial of patients with MRSA carriage or infection during a hospital stay. Participants were randomized to post-discharge education plus 6 months of serial decolonization with 5 day courses of chlorhexidine baths and nasal mupirocin 2x/month (intervention) vs. education alone (control). Participants had swabs of their nares, throat, skin, and any wound at recruitment and months 1, 3, 6, and 9. Isolates from those completing all visits by June 2013 were sequenced and assayed for single nucleotide polymorphism (SNP) variation. Multiple site isolates from the same visit were compared. Isolates differing by ≤10 SNPs were considered the same. Results 974 MRSA isolates were sequenced from 328 participants, of which 318 (97%) had at least one positive MRSA nasal culture. Of these 318 participants, 36 (11%) were colonized concurrently in both the nares and at least one non-nasal site. Over the course of the study, we collected a total of 88 paired isolates from these 36 participants, 21 pairs from 12 participants in the intervention group and 67 pairs from 24 participants in the control group. We found the same MRSA strain in 80% percent of nares vs. throat isolates (n=35, 95% CI 67-93%), 84% of nares vs. skin isolates (n=40, 95% CI 73-95%), and 78% of nares vs. wound isolates (n=9, 95% CI 51-100%). Clonal complex 8 (USA 300/500) strains were more likely to match than CC5 (USA 100) strains (96% vs. 71%, p=0.04) when comparing nares vs. skin isolates. This difference between CC8 and CC5 strains was also present, albeit not significant, when comparing nares vs. throat isolates (88% vs. 74%, p=0.42). Conclusion We found a high degree of genetic relatedness between MRSA strains isolated concurrently from nasal and non-nasal body sites on the same patient, but strains isolated from the skin were more likely to differ from those isolated from the nares for hospital-associated MRSA (CC5) compared to communityassociated MRSA (CC8). Risk Factors for Persistent Colonization with Methicillin-Resistant Staphylococcus aureus Valerie C Cluzet, MD1, Pam Tolomeo, MPH1, Jeffrey S Gerber, MD, PhD, MSCE2, Susan E. Coffin, MD, MPH2, Theoklis E Zaoutis, MD, MS2, Irving Nachamkin, DrPH, MPH1, Warren B. Bilker, PhD1 and Ebbing Lautenbach, MD, MPH, MS1, (1)University of Pennsylvania Perelman School of Medicine, Philadelphia, PA, (2)Perelman School of Medicine at the University of Pennylvania and the Children's Hospital of Philadelphia, Philadelphia, PA Background Colonization with methicillin-resistant Staphylococcus aureus (MRSA) can be classified as intermittent or persistent. Persistent carriers have a higher risk of developing infection and may serve as a source of transmission. Objective To identify factors associated with persistent MRSA colonization Methods We conducted a prospective cohort study between 1/1/2010 and 12/31/2012 at five adult and pediatric academic medical centers. Adults and children presenting to ambulatory settings with an acute community-onset MRSA skin and soft tissue infection (SSTI) (i.e. index cases), along with their household members, performed self-sampling for MRSA colonization every two weeks for six months. Clearance of colonization was defined as two consecutive sampling periods with negative cultures. Subjects who did not meet this definition by the end of the study period were considered persistently colonized. Subjects with persistent MRSA colonization were compared to those without on the basis of demographics, antibiotic exposure and household member colonization status at baseline. Factors associated with persistent colonization were determined using logistic regression analysis. Results A total of 243 index cases were enrolled, of which 48 (19.8%) met the definition of persistent colonization. In multivariable analyses, persistent colonization was associated with older age (odds ratio (OR) for each 10 years, 1.19, 95% confidence interval (CI), 1.03-1.37), prescription of mupirocin within 14 days of SSTI diagnosis (OR, 2.38, 95% CI, 1.08-5.27) and increased proportion of household members with MRSA colonization at baseline (OR for each 10%, 1.15, 95% CI, 1.02-1.29). Conversely, subjects with persistent colonization were less likely to have been treated with clindamycin for the MRSA SSTI (OR, 0.32, 95% CI, 0.15-0.70). Conclusions Nearly 20% of patients with MRSA SSTI will remain persistently colonized. MRSA colonization among household members may contribute to persistence of colonization, indicating a possible role for total household decolonization. The specific effect of clindamycin on MRSA colonization needs to be further elucidated. Determination of Risk Factors for Recurrent Methicillin-resistant Staphylococcus aureus Bacteremia in a Veterans Affairs Healthcare System Population Justin Albertson, MS1, Jennifer McDanel, PhD2, Ryan Carnahan, PharmD MS1, Elizabeth Chrischilles, PhD1, Eli Perencevich, MD, MS3, Michihiko Goto, MD, MSCI4, Lan Jiang, MS5, Bruce Alexander, PharmD5 and Marin Schweizer, PhD6, (1)University of Iowa, Iowa City, IA, (2)601 Highway 6 West 152, University of Iowa, Iowa City, IA, (3)Iowa City VA Medical Center, Iowa City VA Medical Center, Iowa City, IA, (4)SW54-GH, University of Iowa Hospitals and Clinics, Iowa City, IA, (5)Iowa City VA Medical Center, Iowa City, IA, (6)University of Iowa Carver College of Medicine, Iowa City, IA Background Recurrent methicillin-resistant Staphylococcus aureus (MRSA) bacteremia is associated with significant morbidity and mortality and is a potential target for quality improvement. We aimed to identify important risk factors for recurrent MRSA to assist clinicians in identifying high-risk patients for continued surveillance and follow-up. Method In this retrospective cohort study, we examined patients with MRSA bacteremia at 122 VA medical facilities from 2003 to 2010. Recurrent bacteremia was identified by a positive blood culture between 2 days and 180 days after index hospitalization discharge. Subset analyses were performed to evaluate risk factors for early-onset recurrence (2-60 days after discharge) and late-onset recurrence (61-180 days after discharge). An additional subset analysis was performed in which the time period of recurrence was limited to 7-180 days after the index discharge. Risk factors were evaluated using Cox proportional hazards regression. Results A total of 1,159 (6.3%) out of 18,425 patients had recurrent MRSA bacteremia. The median time to recurrence was 63 days. Longer duration of index bacteremia, increased severity of illness, receipt of only vancomycin, community-acquired infection, and several comorbidities were risk factors for recurrence. Congestive heart failure, hypertension, and rheumatoid arthritis/collagen disease were risk factors for early-onset recurrence but not late-onset recurrence. Geographic region and cardiac arrhythmias were risk factors for late-onset recurrence but not early-onset recurrence. Results did not change when the limited time period of recurrence was used. Conclusion Risk factors for recurrent MRSA bacteremia included comorbidities, severity of illness, duration of bacteremia and receipt of only vancomycin. Awareness of risk factors may be important at patient discharge for implementation of quality improvement initiatives including surveillance, follow-up, and education for high-risk patients. May 16, 2015 3:30 - 4:30pm Nutcracker Ballroom 3 Healthcare-Associated Infections in U.S. Nursing Homes: Results from a Prevalence Survey Pilot Lisa LaPlace, MPH1, Lauren Epstein, MD1, Deborah Thompson, MD, MSPH, FACPM2, Ghinwa Dumyati, MD3, Cathleen Concannon, MPH4, Gail Quinlan, RN, MS, CIC5, Jane Harper, MS, RN6, Linn Warnke6, Ruth Lynfield, MD6, Meghan Maloney, MPH7, Richard Melchreit, MD7, Nimalie D. Stone, MD, MS1 and Nicola Thompson, PhD, MS1, (1)Centers for Disease Control and Prevention, Atlanta, GA, (2)New Mexico Department of Health, Santa Fe, NM, (3)Department of Health, University of Rochester, Rochester, NY, (4)Emerging Infections Program, Rochester, NY, (5)CCH - Emerging Infections Program, Rochester, NY, (6)Minnesota Department of Health, Saint Paul, MN, (7)Connecticut Department of Public Health, Hartford, CT Background The national burden of healthcare-associated infections (HAIs) among residents of US nursing homes (NHs) is unknown. Lack of detailed medical record documentation and surveillance capacity are barriers to performing infection surveillance in NHs. Our objective was to pilot the point prevalence survey (PPS) method for HAI surveillance using the CDCs Emerging Infections Program (EIP). Methods A single-day PPS of HAIs was conducted in 9 NH from 4 EIP sites. In each NH, facility staff collected resident demographic and risk factor data on the survey date. EIP staff conducted retrospective chart reviews of residents to collect lab test data, and infection signs and symptoms. HAIs were defined using revised 2012 McGeer definitions. HAI prevalence per 100 residents and by resident characteristics were calculated. Results Among 1272 eligible residents (median age 85 years, 30% male, 14% short stay, 8% device use), the HAI prevalence was 5.3 per 100 residents (95% CI 4.0-6.4%) with 67 residents having 70 HAIs. Most were gastrointestinal tract (n= 26; 37%), skin, soft tissue and mucosal (n= 21; 30%), or respiratory tract (n=16; 23%) infection. Most HAIs (70%) were in long-stay residents without devices, but HAI prevalence was higher in short-stay residents and those with devices (Table). Device use was 3-times higher among short-stay (19.1%) than long-stay residents (6.2%); when stratified by resident stay, device use remained associated with HAIs in short-stay (p=0.016), but not long-stay residents (p=0.549). Resident characteristic, n Stay: Long, 1089 Short, 183 Device: No, 1170 Yes*, 102 Indwelling urinary catheter, 55 Vascular device, 25 Ventilator or tracheostomy, 3 PEG/J tube, 30 HAI prevalence 4.7 8.7 4.8 10.8 16 16 0 0 X-square p-value 0.031 0.018 Conclusion Given a prevalence of 5.3% there is a large burden of HAIs among the ~1.5 million residents receiving care in NHs each day. Device use was significantly associated with HAIs in short-stay but not long-stay residents, indicating the epidemiology and prevention of HAIs in these groups differs. These findings will be used to inform surveillance efforts to estimate HAI burden in NHs. Transmission of MRSA to Healthcare Personnel Gowns and Gloves during Care of Nursing Home Residents Mary-Claire Roghmann, MD, MS1, J. Kristie Johnson, PhD1, John D. Sorkin, MD, PhD1, Patricia Langenberg, PhD1, Alison Lydecker, MPH1, Brian Sorace, BS1, Lauren Levy, JD, MPH1and Lona Mody, MD, MSc2, (1)University of Maryland School of Medicine, Baltimore, MD, (2)University of Michigan School of Medicine, Ann Arbor, MI Objective To estimate the frequency of MRSA transmission to gowns and gloves worn by healthcare personnel (HCP) interacting with nursing home residents in order to inform infection prevention policies in this setting Design Observational study Setting and Participants Residents and HCP from 13 community-based nursing homes in Maryland and Michigan Methods Residents were cultured for MRSA at the anterior nares and perianal or perineal skin. HCP wore gowns and gloves during usual care activities. At the end of each activity, a research coordinator swabbed the HCP’s gown and gloves. Results 403 residents were enrolled; 113 were MRSA colonized. Glove contamination was higher than gown contamination (24% vs. 14% of 954 interactions, p<0.01). Transmission varied greatly by type of care from 0% to 24% for gowns and 8% to 37% for gloves. We identified high risk activities (OR >1.0, p< 0.05) including: dressing, transferring, providing hygiene, changing linens and toileting the resident. We identified low risk activities (OR <1.0, p< 0.05) including: giving medications and performing glucose monitoring. Residents with chronic skin breakdown had significantly higher rates of gown and glove contamination. Conclusions MRSA transmission from MRSA positive residents to HCP gown and gloves is substantial with high contact activities of daily living conferring the highest risk. These activities do not involve overt contact with body fluids, skin breakdown or mucous membranes suggesting the need to modify current standards of care involving the use of gowns and gloves in this setting. Risk Factors for Infection or Colonization with Carbapenem-Resistant Klebsiella pneumoniae in a Longterm Acute Care Hospital John P Mills, MD1, Naasha J Talati, MD, MSCR1, Kevin Alby, PhD2 and Jennifer Han, MD, MSCE1, (1)University of Pennsylvania, Philadelphia, PA, (2)University of Pennsylvania Health System, Philadelphia, PA Background Carbapenem-resistant Klebsiella pneumoniae (CRKP) infections are associated with high mortality rates and have become increasingly prevalent in long-term acute care hospitals (LTACHs). Few data exist on the epidemiology of CRKP in the LTACH setting. The objective of this study was to identify risk factors for infection or colonization with CRKP in LTACH patients. Methods A case-control study was conducted in a university-affiliated LTACH from July 2008 to July 2014. All patients with clinical cultures positive for CRKP were included as cases, and all patients with clinical cultures with carbapenem-susceptible Klebsiella pneumoniae served as controls. A multivariable logistic regression model was developed to identify risk factors for infection or colonization with CRKP. Results Ninety-nine (45%) of 222 patients with a K. pneumoniae clinical culture during the six-year study period were colonized or infected with CRKP. The median age of study patients was 71 years and 48% were male. Mean length of stay prior to culture isolation was 12.3 days and 9.3 days for cases and controls, respectively (P=0.32). The source of culture for cases were: 56 (57%) respiratory, 40 (40%) urine, 3 (3%) blood. The source of culture for controls were: 69 (56%) urine, 42 (34%) respiratory, 10 (8%) blood, and 2 (2%) wound. On multivariable analyses, the following risk factors for CRKP infection or colonization were identified: solid organ or hematopoietic stem cell transplantation (odds ratio [OR] 5.1, 95% confidence interval [CI] 1.22 – 20.79, P=0.03), mechanical ventilation (OR 2.6, 95% CI 1.24 – 5.28, P=0.01), fecal incontinence (OR 5.8, 95% CI 1.52 – 22.02, P=0.01), meropenem use (OR 3.6, 95% CI 1.04 – 12.07,P=0.04), vancomycin use (OR 2.9, 95% CI 1.18 – 7.32, P=0.02), and metronidazole use (OR 4.2, 1.28 – 13.95, P=0.02). Conclusion Nearly half of all study LTACH patients with infection or colonization with K. pneumoniae had cultures with CRKP. Patients with prior transplantation, mechanical ventilation, fecal incontinence, and exposure to meropenem, vancomycin, and metronidazole represent a population in LTACHs at increased risk for infection or colonization with CRKP. These high-risk groups may represent populations who merit active surveillance for CRKP. Designing a ‘change-score' metric to characterize antibiotic resistant Gram-negative bacilli (R-GNB) colonization in post-acute care facilities Sara E McNamara, MPH1, Mohammed Kabeto, MA1, Lillian Min, MD1 and Lona Mody, MD2, (1)University of Michigan, Ann Arbor, MI, (2)University of Michigan and Ann Arbor VA Healthcare System, Ann Arbor, MI Background Prevalence of resistant Gram-negative bacilli (R-GNB) surpasses methicillin-resistant Staphylococcus aureusand vancomycin-resistant enterococci rates in post-acute and long-term care facilities, particularly among residents with indwelling devices. R-GNB colonization is often transient, and there are many different species of R-GNB, which makes understanding this diverse group of organisms challenging. We sought to design a ‘change-score’ to characterize this dynamic nature of R-GNB colonization. Methods As a part of the Targeted Infection Prevention cluster-randomized study, we evaluated the presence of R-GNB among residents with indwelling devices in 12 facilities. Cultures from multiple anatomic sites were obtained at enrollment, at15 days, and every 30 days thereafter. The gain or loss of each R-GNB species at each follow-up visit was scored as 1 change. The change-score was calculated as the total number of changes occurring during follow-up. Results 304 residents with indwelling devices were cultured at more than 1 visit (34,567 follow-up days). 112 (37%) residents had no change in R-GNB colonization during follow-up (100 (33%) were never colonized, 12 (4%) were persistently colonized over the entire follow-up); 102 (34%) had 1 to 3 R-GNB changes, and 90 (30%) had ≥4 changes. Residents with ≥4 R-GNB changes were younger, more functionally impaired, had a longer follow-up time, were more likely to have multiple indwelling devices, a history of R-GNB, and any antibiotic use, infection, hospitalization or wounds during follow-up. Adjusting for facility-level clustering and follow-up time, age (RR 0.98, 95% CI (0.97, 0.99), p<0.001) and functional status (RR 1.06, 95% CI (1.01, 1.11), p=0.01) were associated with R-GNB change-scores. Conclusions We describe a metric to further characterize R-GNB epidemiology in post-acute and long-term care facilities. Using this change-score, we show that R-GNB colonization is dynamic with multiple episodes of R-GNB gain and loss. Common risk factors for R-GNB increased with increasing change-score. This change-score metric may be useful in designing infection prevention strategies in this frail and functionally impaired population with prolonged stays in an institutional setting. May 17, 2015 8:30 - 10:00am Fantasia Ballroom G Impact of Service Age on the Effectiveness of Reprocessing for Flexible Gastrointestinal Endoscopes Marco Bommarito, PhD1,2, Julie Stahl, BS2 and Dan Morse, MS2, (1)3M, St. Paul, MN, (2)3M Infection Prevention Division, St. Paul, MN Background The effectiveness of cleaning and disinfecting patient-used endoscopes was characterized by measuring: Adenosine Tri-Phosphate (ATP), colony forming units (CFUs), and protein. The study included two phases and an intervention. The intervention consisted of replacing all scopes in the inventory (in service for many years) with brand new devices. Method For both phases, the concentrations of three markers in a total of 27 colonoscopes and 28 gastroscopes reprocessed (using the same protocol) in the endoscopy suite of an East Coast Hospital were measured at three points during reprocessing: pre-manual cleaning (after bedside flushing), post-manual cleaning, and after high level disinfection (HLD). ATP levels inLog(RLUs) (Relative Light Units) were determined using a luminometer. Protein concentrations in mg/mL were determined using a BCA assay. Total aerobic Log(CFUs) were determined by culture. Result Figure 1 (Phase 1, old endoscopes) and Figure 2 (Phase 2, new endoscopes) show the mean amounts of ATP in Log(RLUs) (A), Log(CFUs) (B) and protein in mg/mL (C) found for each reprocessing step by type of scope. Also shown are the Log reductions for the ATP and CFU components achieved for manual cleaning and HLD (D, asterisks show statistically significant reductions). Error bars are one standard error and raw data values are in parenthesis. After manual cleaning, Phase 2 endoscopes had 0.58 logs lower ATP levels than Phase 1 endoscopes (p<0.0001), similarly Phase 2 scopes had 0.94 logs lower levels of CFUs contamination compared to scopes in Phase 1 (p<0.0001). Furthermore, 95% of the endoscopes were culture positive after manual cleaning in Phase 1 compared to 68% in Phase 2. After HLD, Phase 2 scopes had 0.44 logs lower ATP levels than Phase 1 endoscopes (p<0.0001). 14% of the endoscopes were culture positive after HLD in Phase 1 compared to 9% in Phase 2. Protein results were highly correlated with the ATP results for each process step. Conclusion Replacing endoscopes after many years of service with brand new devices resulted in significant improvements in the effectiveness of reprocessing indicating that the age of a device is a very significant variable in the outcome of this workflow. Defining Ventilator Associated Conditions in Neonates & Children Noelle Cocoros, DSc, MPH1, Ken Kleinman, ScD1, Gregory P. Priebe, MD2, James E Gray, MD, MS3, Gitte Larsen, MD, MPH4, Latania Logan, MD5, Philip Toltzis, MD6, Sarah B. Klieger, MPH7, Michael Klompas, MD1,8 and Grace Lee, MD, MPH1,9, (1)Harvard Pilgrim Health Care Institute & Harvard Medical School, Boston, MA, (2)Children's Hospital Boston, Boston, MA, (3)Beth Israel Deaconess Medical Center, Boston, MA, (4)University of Utah Healthcare, Salt Lake City, UT, (5)Rush University Medical Center, Chicago, IL, (6)Rainbow Babies & Children's Hospital, Cleveland, OH, (7)Children's Hospital of Philadelphia, Philadelphia, PA, (8)Brigham and Women's Hospital, Boston, MA, (9)Boston Children's Hospital, Boston, MA Background National surveillance definitions for ventilator-associated conditions (VAC) in adults were developed and implemented beginning in 2013. We sought to develop a pediatric VAC (PVAC) definition suitable for use in neonates and children by exploring the ability of potential definitions to discriminate patients with worse outcomes. Methods We retrospectively identified consecutive children ≤18 years ventilated for ≥1 day in pediatric, cardiac or neonatal ICUs in 5 US hospitals. We evaluated alternative thresholds for increases in daily minimum FIO2 (by 0.20, 0.25, 0.30) or daily minimum mean airway pressure (MAP) (by 4, 5, 6, 7 cm H20). As with the adult definition, we sought increases sustained for ≥2 days after ≥2 days of stable or decreasing settings. We matched up to 4 patients without a PVAC to each patient with a PVAC and used Cox proportional hazard models with frailties to estimate the association between hospital mortality, length of stay and duration of ventilation with PVAC, stratified by ICU type. Results Our cohort included 10,209 hospitalizations and 77,751 ventilator days from 4 NICUs, 4 PICUs and 3 CICUs. All PVAC definitions were significantly associated with greater risk for death. Hazard ratios (HR) for death varied from 1.6 to 6.8 depending on thresholds and ICU type. PVAC was also associated with prolonged hospitalization and ICU stay, particularly for PICU and CICU patients, and lower hazard for extubation (i.e. longer duration of ventilation; HR for extubation, 0.23 to 0.59) among survivors. For the FIO20.20/MAP4 definition (i.e. increase in minimum daily FIO2 by 0.20 or MAP by 4), PVAC rates in the full cohort ranged from 3.3 to 4.6 per 1,000 ventilator days depending on the ICU type; in comparison, the FIO20.30/MAP7 definition yielded PVAC rates of 1.1 to 1.3 per 1,000 ventilator days. Conclusions Pediatric patients with PVAC are at substantially higher risk for mortality and morbidity in PICUs, CICUs and NICUs, regardless of thresholds used. Tradeoffs in sensitivity, specificity and opportunities for improvement will need to be considered when selecting a PVAC definition for use in national surveillance. Genomic analysis demonstrates that decolonization with chlorhexidine and mupirocin reduces both colonization with existing MRSA strains and re-colonization with new MRSA strains Michael S. Calderwood, MD, MPH1,2, Christopher A. Desjardins, PhD3, Michael Feldgarden, PhD4, Yonatan Grad, MD PhD1,5, Diane Kim, BS6, Raveena Singh, MS6, Samantha J. Eells, MPH7,8, James A. McKinnell, MD7,9,10, Steven Park, MD PhD6, Ellena Peterson, PhD11, Loren G. Miller, MD MPH7,10 and Susan S. Huang, MD MPH6, (1)Brigham and Women's Hospital, Division of Infectious Diseases, Boston, MA, (2)Harvard Medical School and Harvard Pilgrim Health Care Institute, Department of Population Medicine, Boston, MA, (3)Broad Institute of MIT and Harvard, Cambridge, MA, (4)National Center for Biotechnology Information, National Institutes of Health, Bethesda, MD, (5)Center for Communicable Disease Dynamics, Department of Epidemiology, Harvard School of Public Health, Boston, MA, (6)Division of Infectious Diseases and Health Policy Research Institute, University of California, Irvine School of Medicine, Irvine, CA, (7)Infectious Disease Clinical Outcomes Research Unit (ID-CORE), Division of Infectious Disease, Los Angeles Biomedical Research Institute at Harbor-University of California Los Angeles (UCLA) Medical Center, Torrance, CA, (8)Department of Epidemiology, UCLA Fielding School of Public Health, Los Angeles, CA, (9)Torrance Memorial Medical Center, Torrance, CA, (10)David Geffen School of Medicine, Los Angeles, CA, (11)Department of Pathology and Laboratory Medicine, University of California, Irvine School of Medicine, Irvine, CA Background MRSA carriers are at higher risk of MRSA infection. Decolonization with chlorhexidine and mupirocin decreases MRSA colonization. Methods We examined the genetic relatedness of MRSA nasal isolates collected from Project CLEAR, a randomized clinical trial of recently hospitalized MRSA carriers and MRSA infected patients. Participants were randomized to education alone or education plus 6 months of serial (twice monthly) decolonization with 5 days of chlorhexidine bathing and nasal mupirocin, and had multiple sites swabbed for MRSA culture at recruitment and follow-up visits at 1, 3, 6, and 9 months. We analyzed nasal isolates from patients who completed all visits through June 2013. MRSA isolates underwent whole genome sequencing and were assayed for single nucleotide polymorphism (SNP) variation. Isolates that differed by ≤10 SNPs were considered to represent the same MRSA strain. Fisher's exact tests were used to compare differences between groups. Results We found MRSA nares colonization at enrollment in 112/235 (48%) of the intervention group and 130/244 (53%) of the control group (p = 0.24). At the 1-month visit, 18 (16%) in the intervention group were colonized with the same MRSA strain in their nares, compared with 53 (41%) in the control group (p <0.01, Figure). In addition, 7% of patients in both groups were colonized with a new MRSA strain (8 in the intervention group, 10 in the control group). By the 6-month visit, 7 (6%) in the intervention group were colonized with the same MRSA strain in their nares, compared with 37 (28%) in the control group (p <0.01). The number of patients colonized with a new MRSA strain was 10 (9%) in the intervention group, compared with 26 (20%) in the control group (p-value 0.02). There was no difference in samestrain nasal carriage in the intervention group 3 months after stopping the decolonization intervention (13 patients at month 9 vs. 7 patients at month 6, p-value 0.24). Conclusion Decolonization with chlorhexidine/mupirocin reduced same strain MRSA nares colonization compared to controls in a long-term trial of MRSA carriers. No significant rebound in same-strain colonization occurred 3 months after stopping decolonization. In addition, decolonization reduced nares colonization with new MRSA strains. Comparison of explicit criteria for determining appropriateness of antibiotic prescribing in nursing homes Christopher Crnich, MD, PhD1,2, Jill Miller1, Mozhdeh Bahrainian, MS1 and Sowmya Adibhatla1, (1)University of Wisconsin School of Medicine & Public Health, Madison, WI, (2)William S. Middleton Memorial VA Hospital, Madison, WI Background Antibiotic use is a major driver of antibiotic resistance in nursing homes (NHs) and there is increasing interest in understanding how much antibiotic overuse contributes to this problem. Many studies of appropriateness of antibiotic prescribing in NHs have employed rule-based criteria. The explicit criteria most commonly employed in these studies are the McGeer and Loeb criteria. To our knowledge, level of agreement between these two sets of criteria is unknown. Method We performed a comparative analysis of the McGeer and Loeb criteria using data abstracted from the health records in five community NHs. Antibiotic courses initiated in the hospital and emergency department (ED) were excluded from further analysis. Appropriateness of antibiotics initiated for a urinary tract infection (UTI), respiratory tract infection (RTI) and skin/soft tissue infection (SSTI) indication was assessed separately using both the McGeer and Loeb criteria. The overall level of agreement between the two criteria was assessed using kappa statistics, stratified by indication for initiating antibiotics (UTI vs. RTI vs. SSTI). Result 524 of the observed 1108 antibiotic courses (47.3%) were initiated in the hospital or ED. These events, as well as 60 antibiotic courses (5.4%) prescribed for non-applicable conditions, were excluded from further analysis. Of the 504 evaluable antibiotic courses initiated in the NH, resident signs and symptoms meeting either the McGeer or Loeb criteria were documented in less than half the cases (48.2%). Overall level of agreement between the two sets of explicit criteria was low (k = 0.35) but varied by prescribing indication: 1) UTI (k = 0.56); 2) SSTI (k = 0.35); and 3) RTI (k = 0.258). The greatest discrepancy in appropriateness was seen with SSTI where 68% of events were appropriate when using Loeb criteria but only 31% were appropriate when using McGeer criteria. Conclusion Documentation supporting the appropriateness of antibiotic prescribing in NHs is frequently absent, regardless of explicit criteria employed. However, overall level of agreement between the McGeer and Loeb criteria is low. Delineating which set of criteria has greater utility for monitoring quality of antibiotic prescribing in NHs requires additional study. Comparing hospital onset bacteremia to Central Line-Associated Bloodstream infection as a Hospital Quality Measure; is it Time for a New Quality Measure? Clare Rock, MD, MS1, Kerri Thom, MD, MS2, Anthony Harris, MD, MPH2, Shanshan Li, PhD3, Daniel Morgan, MD, MS2, Aaron Milstone, MD MHS4, Brian Caffo, PhD5 and Surbhi Leekha, MBBS, MPH2, (1)Department of Medicine, Division of Infectious Diseases, Johns Hopkins University, Baltimore, MD, (2)Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, (3)Department of Biostatistics, Indiana University Fairbanks School of Public Health, Indianapolis, IN, (4)Johns Hopkins University, Baltimore, MD, (5)Department of Biostatistics, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, MD Background Central line associated bloodstream infection (CLABSI) is an important quality measure tied to hospital reimbursement, yet the measure suffers from subjectivity and inter-rater variability. Further, with decreases in CLABSI nationally, its power to discriminate between hospitals is potentially compromised. A common scenario is hypothetical Hospital A’s reported CLABSI Standardized Infection Ratio (SIR) of 1.2 (95% Confidence Interval (CI) 0.8, 2.1), and Hospital B’s CLABSI SIR of 0.8 (95% CI 0.5, 1.3). With these large overlapping CIs, how is it possible to discern if these SIRs and hospital infection prevention performance are different from each other? The objective of this study was to evaluate hospital-onset bacteremia (HOB), i.e., any positive blood culture obtained 48 hours post-admission, as a substitute for CLABSI. We assessed the association between CLABSI and HOB rates in ICUs, and their power to discriminate between ICUs. We hypothesized that HOB is associated with CLABSI, and has greater power to discriminate between ICUs. Methods We conducted a multisite cohort study via the SHEA research network. Hospitals performing ICU CLABSI surveillance provided monthly CLABSI and HOB rates for 2012 and 2013. A Poisson regression model was used to assess the association between the two rates. We compared the power of the two measures to discriminate between ICU using SIRs with 95% CIs. A measure was defined as having greater power to discriminate if more of the SIRs (with surrounding CIs) were different from 1 (the benchmark SIR). Results 80 ICUs from 16 hospitals in the US and Canada reported a total of 663 CLABSIs, 475,420 central line days, 11,280 HOB and 966,757 ICU patient days. HOB was strongly associated with CLABSI; an absolute change in HOB of 1 / 1,000 ICU patient days was associated with a 2.5% change in CLABSI rate (P<0.001). For the 18 medical and neonatal ICUs in our study, the CLABSI SIR was different from 1 in 4/18 cases (22%), compared to 12/18 cases (67%) for HOB (Fisher’s exact P= 0.02). Conclusions HOB and CLABSI rates are strongly associated. HOB has advantages over CLABSI including a greater power to discriminate between ICU performances. Consideration should be given to using HOB as an outcome measure in infection prevention quality.