Effect of humic and fulvic acids on the photocatalytic degradation of
advertisement

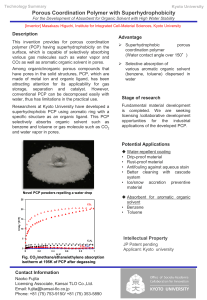
17th-IHSS Conference September 1-5, 2014 Ioannina, Greece The Dual Role of Humic Acid on Catalytic Decomposition of Pentachlorophenol by the Iron-Fenton System A. Smith (a) *, B. White(b), C. Rice(b) (a) Address a (b) Address b. * Corresponding author e-mail: xxxxxx@yyyyyy Keywords: up to 6 keywords Abstract [UP TO 150 WORDS] The capacity of Fenton [Fe/H2O2] on the catalytic decomposition of pentachlorophenol (PCP) in the presence and absence of humic acid (HA) was evaluated. Numerous studies have shown that HA can act either as an enhancer or inhibitor of the Fenton reaction. Herein, by combining Electron Paramagnetic Resonance (EPR) spectroscopic and catalytic data, it is shown that HA can act in a dual manner e.g. either as an enhancer or as an inhibitor for PCP oxidation depending on the initial Fe/HA ratio. A quantitative limit is established: [i] at low Fe/HA < 1.17 mmol/gr the presence of HA resulted to a decrease of PCP decomposition while [ii] at high Fe/HA > 1.17 mmol/gr a significant enhancement was observed. EPR spectroscopy revealed that, HA acted [i] as a chelator of Fe and [ii] as a redox agent.. Introduction [UP TO 350 WORDS] Pentachlorophenol (PCP) is poorly biodegradable, highly toxic therefore considerable research efforts have been devoted to the development of abiotic catalytic methods e.g. dechlorination by metal catalysts and Advanced Oxidation Processes. The Fenton system (FeII/ H2O2) offers tha basis for a ccost –effective technology for the degradation of a broad range of organic pollutants. Hydroxyl radical (HO˙) is formed during the catalytic decomposition of H2O2 by iron salts. HO˙ is a highly reactive, nonselective radical (1). Since HO˙ is nonselective, in natural aquatic samples it may react with dissolved natural organic matter (NOM) i.e. beyond organic pollutants. In this way NOM can be a significant sink of HO˙ resulting to a considerable decrease of the reaction’s efficiency. Moreover, Fe-binding by compounds, such as NOM, humic (HA) and fulvic acids (FA), can also alter the rate constant for the reaction or the redox cycle of iron and thereby change the formation rate of HO˙ . It has been demonstrated that in a catalytic Fenton system consisting of Fe, HA and H2O2, HO˙ radicals are produced (2). However, the role of HA on the HO˙ radicals appears controversial e.g. HA has been reported to either increase (3) or decrease (4) the production of HO˙. To add to this complexity, recently it has been reported that the initial catalytic conditions may influence HO˙ production (5). The effect of HA on the catalytic efficiency of the Fenton reaction still remains controversial since the degradation of organic pollutants has been reported to be either inhibited (6) or enhanced (7) in the presence of humic materials. All these cases are summarized in ref. 8. The aims of the present work were (a) to examine the role of a well characterized HA- on the catalytic decomposition of PCP by the Fenton system, (b) to frame quatitative limits regarding the Fe/HA ratio for optimal yield/rate of PCP decomposition, (c) to study the physicochemical properties of Fe in the catalytic Fenton/PCP system by EPR spectroscopy. Experimental The low-Fe HA used was a well characterised-HA sample extracted from a mining site of Greece according to the protocols of IHSS. All reactions were conducted at room temperature and in dark to exclude adverse photo-effects. The pH was adjusted to 3.5 using H2SO4 e.g. since production of HO˙ by Fenton reaction declines at higher pH, in the presence or absence of humic materials. A typical reaction mixture contained 13ppm PCP, 0.87-32.5ppm FeSO4∙7H2O, 4.35-97ppm H2O2 and 20 ppm HA. The effect of [H2O2], [Fe] and the presence of 20ppm HA was studied (Table 1). Detailed control experiments were performed using solutions containing [PCP+FeSO4∙7H2O] or [PCP+H2O2] or [PCP+FeSO4∙7H2O+HA] or [PCP+H2O2+HA]. All these systems resulted to zero conversion of PCP. Results and Discussion Fenton Catalyst in the Absence of HA:[Figure 1A] Fe-concentration appeared to have a decisive effect on PCP degradation. For example, by increasing the FeSO4∙7H2O content to 6.5ppm, the total of PCP was removed within 120 h (compare Fig. 1A, B). Increase of [H2O2] did not affect significantly the degradation of PCP (Figure S1A). For example, increase of [H2O2] by 2200% i.e. 22 times, (run 1 vs. 5) resulted to an increase of PCP removal by 18%. September 1-5, 2014 Ioannina, Greece 80 0.87 ppm % [PCP] Remained 40 20 0 dX''/dH (au) [FeSO47H2O] 60 run 16 (e) run 19 0 runs 11-15 1500 3000 [HA] ppm 4500 (d) Signal Intensity A A runs 4, 6-10 100 g=4.3 EPR Signal Intensity 17th-IHSS Conference (c) 32.5 ppm runs 14, 16-20 100 B (b) (a) [FeSO47H2O] 0.87 ppm 80 400 60 800 1200 1600 2000 2400 Magnetic Field (G) 40 40 B runs 11-15 20 32.5 ppm 0 72 144 216 time (h) Figure 1. Effect of [FeSO4∙7H2O] on the removal of PCP by Fenton reaction (A) in the absence and (B) in the presence of 20ppm HA. Catalytic conditions: 13 ppm PCP, 52 ppm H2O2, 0.87-32.5 ppm FeSO4∙7H2O. Run 4 and 14 (), run 6 and 16 (), run 7 and 17 (), run 8 and 18 (), run 9 and 19 (), run 10 and 20 (). % [Fe-HA ] 30 0 run 16 20 RFe/HA=1.17 10 run 19 0 3.6 HA-Modified Fenton Catalyst: [Figure 1B] FeII concentration had a prominent effect i.e. depending on the initial [FeII]:HA ratio. Noticeably, in the presence of HA, increase of H2O2 concentration had a beneficial effect which was higher than the effect of H2O2 in the absence of HA: [i] under the conditions of these reactions, HA inhibited PCP decomposition; [ii] in the presence of HA, more [H2O2] oxidant was required to achieve the same PCP decomposition as in the absence of HA [iii] further increase of [H2O2] resulted to a linear increase of the total PCP conversion at 9 days in the presence or absence of HA. Overall, under the conditions of our experiments: [i] HA appears to play a dual Janus-like role e.g. HA can act either as an inhibitor or as an enhancer of the Fenton reaction, on both PCP decomposition yield and reaction rates; [ii] the function of HA is gated by the ratio Fe/HA (RFe/HA). When Fe(mol) R Fe/HA 1.17 HA(mg) both PCP decomposition yield and reaction rates were increased otherwise they remained constant or decreased vs. the unmodified Fenton. EPR Spectroscopy [Figure 2A] shows the percent of monomeric FeIII-HA and the reduced FeII (due to the presence of HA) formed in each sample used for the Fenton catalysis of PCP, i.e. the percent of Fe (FeIII and FeII) interacting with HA (Fe-HA). It is seen that increase of the HA concentration keeping the Fecontent constant (i.e. decrease of RFe/HA ratio) resulted to an increase of the [Fe-HA] species. The downarrow in Figure 4B mark the RFe/HA=1.17, while the horizontal arrows mark the RFe/HA regimes where oxidation of PCP is increased/decreased in the presence of HA (Figure 2B). Increase Decrease of PCP of PCP oxidation oxidation Low RFe/HA High RFe/HA 3.0 2.4 1.8 1.2 0.6 Fe/HA (mol/mg) Figure 2. A) EPR spectra of 0.4 mM Fe2(SO4)3∙xH2O incubated in the absence of HA, spectrum (a) and (b) (spectrum (b) has 30 % v/v glycerol) and in the presence of (c) 230, (d) 1710 and (e) 5110 ppm HA (i.e. corresponding to RFe/HA = 0.16, 0.47 and 3.5 μmol/mg respectively) for 2 h at pH 3.5. Inset plot: EPR FeIII signal intensity as a function of [HA] incubated for t 30 min () and 2 h (), the lines correspond to a linear regression of the data. B) Percent of the [Fe-HA] after incubated for 2 h at pH 3.5. Each case is labelled with the corresponding Fenton reaction with the same ratio RFe/HA REFERENCES [UP to 8 references] (1) Rice, N.; Buxton, G.V; Nixon, A.B. J. Phys. Chem. Ref. Data 1988, 17, 513-886. (2) Paciolla, M.D. and White A.B. Environ. Sci. Technol. 1999, 33, 1814-1818. (3) Huling, S.G.; Water Res. 2001, 35, 1687-1694 (4) Lindsey, M.; Tarr, M.A.. Chemosphere 2000, 41, 409-417. (5) Ciotti, C.; Baciocchi, R.; Tuhkanen, T J. Hazard. Mater. 2009, 161, 402-408. (6) Lindsey, M.; Tarr, M.A. Environ. Sci. Technol. 2000, 34, 444-449. (7) Fukushima, M.; Tatsumi, K. Environ. Sci. Technol. 2001, 35, 1771-1778. (8) Georgi, A. Appl. Catal. B: Environ. 2007, 72, 26-36. Acknowledgments: xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx xxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxxx