Word Format - WACE 2015 2016
advertisement
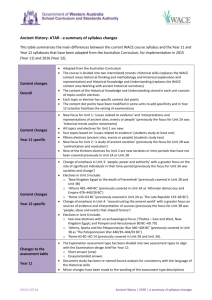
Chemistry: ATAR - a summary of syllabus changes This table summarises the main differences between the current WACE course syllabus and the Year 11 and Year 12 syllabuses that have been adapted from the Australian Curriculum, for implementation in 2015 (Year 11) and 2016 (Year 12). Content changes Overall The Chemistry ATAR course has three interrelated strands: Science Inquiry Skills, Science as a Human Endeavour and Science Understanding which build on students’ learning in the P-10 Australian Curriculum: Science. The content descriptions for Science Inquiry Skills, Science as a Human Endeavour and Science Understanding have been written so that integration of the strands is possible in each unit Mathematical skills expected of students studying Chemistry are explicated. It is recognised that students may need to be taught to interpret logarithmic scales and to substitute a value to evaluate a logarithmic expression as they are required in pH calculations (Unit 3) The syllabus also incorporates two new sections, ‘Representation of General capabilities’ and ‘Representation of Cross-curriculum priorities In the new course, Units 1–3 all Australian curriculum content have been included. Unit 3 includes calculation of hydrogen or hydroxide ion concentration from K w; calculation of cell potential, and electrolysis In Unit 4, the following content included: polymers to include polyethene, polytetrafluoroethene, polyamides and polyester; amides, protein structure, Haber and Contact processes, and enzyme catalysis Content changes Year 11 specific Content from the current WACE Stages 2A and 2B (excluding oxidation and reduction) are included in Units 1 and 2 but also includes Atomic structure and bonding content from Stage 3A. Unit 1: Chemical fundamentals: structure, properties and reactions Models of atomic structure and bonding to explain the macroscopic properties of materials, properties of nanomaterials now included. The energy changes associated with chemical reactions and the use of chemical equations to calculate the masses of substances involved in chemical reactions Unit 2: Molecular interactions and reactions Bonding models and the relationship between structure, properties and reactions, including consideration of the factors that affect the rate of chemical reactions. The unique properties of water and the properties of acids and bases, and use chemical equations to calculate the concentrations and volumes of solutions involved in chemical reactions, identification of ions in aqueous solutions with flame tests now included Content changes Year 12 specific Atomic structure and bonding content in the current Stage 3A is now in Units 1 and 2 (Year 11). Other content in Stages 3A and 3B are in Units 3 and 4. Unit 3: Equilibrium, acids and bases, and redox reactions The concept of reversibility of reactions and the dynamic nature of equilibrium in chemical systems; contemporary models of acid-base behaviour that explain their properties and uses , the use of Kw to calculate hydrogen or hydroxide ion concentration in solutions of strong acids or bases; the principles of oxidation and reduction reactions including the generation of electricity from electrochemical cells and calculation of their cell potentials under standard conditions, and electrolytic cells including metal plating and copper purification Unit 4: Organic chemistry and chemical synthesis The relationship between the structure, properties and chemical reactions of organic functional groups now includes amides, addition polymerisation includes polyethene and polytetrafluoroethene and condensation polymerisation includes polyamides and polyesters, α-amino acids include the formation of zwitterions and their ability to form amide (peptide) bonds, protein primary, secondary and 2014/10311v3 Chemistry | ATAR | a summary of syllabus changes tertiary structures; chemical synthesis processes include the Haber and Contact processes, biodiesel production by base-catalysed and lipase-catalysed methods, the use of enzyme catalysts with fermentation of ethanol contrasted with hydrolysis of ethene; chemical synthesis processes that involve a sequence of reactions Changes to the assessment table Year 11 Type of assessment Science Inquiry—Practical and Investigation types: 25% (current Stage 2 course 15–25%) Extended response: 10% (not represented in the current assessment structure as a separate type) Test: 15% (current course, tests and examinations are conflated and are allocated 50–70% with the disbursement of that component not specified) Examination : 50% Changes to the assessment table Year 12 Type of assessment Science Inquiry—Practical and Investigation types: 20% (current Stage 3 course 15–25%) Extended response: 10% (not represented in the current assessment structure as a separate type) Test: 20% (current course, ‘Tests and examinations’ are conflated and are allocated 50–70% with the disbursement of that component not specified) Examination: 50% Changes to assessment type weightings Year 11 The weighting for the category ‘Science inquiry’ is set at the top end of the range in the current course. The assessment type ‘Test’ is correspondingly reduced. The assessment type ‘Examination’ is unchanged. Changes to assessment type weightings Year 12 The weighting for the category ‘Science inquiry’ for the new course is set at the top end of the range of the current course. The assessment type ‘Test’ is correspondingly reduced. The assessment type ‘Examination’ is unchanged. Examination design brief changes Section/s renamed Nil Section/s format or weighting Minor changes to the specified format of the examination. Weighting of calculations across Sections Two and Three is now 15-20% (formally 15-25%). Note also that numerical answers should be expressed to the appropriate number of significant figures (not three significant figures). Sections removed Nil Sections added Nil Other changes Nil 2014/10311v3 Chemistry | ATAR | a summary of syllabus changes