Key
advertisement
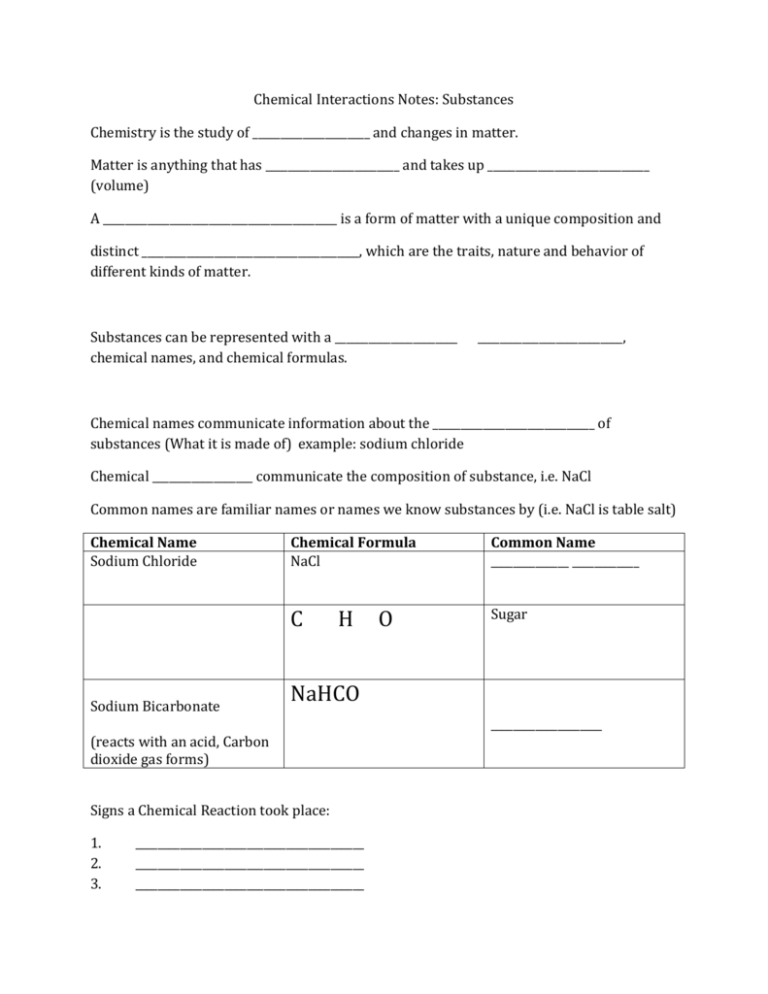
Chemical Interactions Notes: Substances Chemistry is the study of _____________________ and changes in matter. Matter is anything that has ________________________ and takes up _____________________________ (volume) A __________________________________________ is a form of matter with a unique composition and distinct _______________________________________, which are the traits, nature and behavior of different kinds of matter. Substances can be represented with a ______________________ chemical names, and chemical formulas. __________________________, Chemical names communicate information about the _____________________________ of substances (What it is made of) example: sodium chloride Chemical __________________ communicate the composition of substance, i.e. NaCl Common names are familiar names or names we know substances by (i.e. NaCl is table salt) Chemical Name Sodium Chloride Sodium Bicarbonate Chemical Formula NaCl Common Name ______________ ____________ C Sugar H NaHCO (reacts with an acid, Carbon dioxide gas forms) Signs a Chemical Reaction took place: 1. 2. 3. O _________________________________________ _________________________________________ _________________________________________ ____________________ 4. _________________________________________ Chemical Interactions Notes: Substances Chemistry is the study of ______matter_______________ and changes in matter. Matter is anything that has _____mass___________________ and takes up ____________space_________________ (volume) A _________________substance_________________________ is a form of matter with a unique composition and distinct ______properties_________________________________, which are the traits, nature and behavior of different kinds of matter. Substances can be represented with a _____common _________________ _______name___________________, chemical names, and chemical formulas. Chemical names communicate information about the _________composition____________________ of substances (What it is made of) example: sodium chloride Chemical _____formulas______ _______ communicate the composition of substance, i.e. NaCl Common names are familiar names or names we know substances by (i.e. NaCl is table salt) Chemical Name Sodium Chloride sucrose Sodium Bicarbonate Chemical Formula NaCl Common Name _______table salt_______ C Sugar H O NaHCO Signs a Chemical Reaction took place: 1. Gas forms (bubbles) 2. an odor 3. change in color 4. change in temperature _______baking soda Science Notes: Cloze Notes to go with Slide Show An Element is: -an unchangeable _______________________________________ -can not be _______________________________________________ down into simpler subsatnces. -_______________________________________________________________ of all matter ___________________________________________________ has a mass and takes up space. Elements and Matter - Elements _______________________________________________ to form all the different substances (matter) in the world. PROPERTIES Chemical Properties: a chemical property is a property or behavior of a substance when it undergoes a chemical change or ________________________________. Physical Properties: the measurement of a physical property may ______________________________________ the arrangement of matter in a sample, but NOT the _______________________________ of its molecules. Example: water can be frozen, can be a liquid, or a gas but its chemical makeup does not change. Mendelev: Created the ____________________________________________ periodic table in 1869. The _____________________________________________ that were most alike were grouped together in the same _________________________________________________. He called those ____________________________________________________________________________________. After arranging his version of the table, he noticed there were ______________________. This lead him to predict that an _________________________________________________ element would fit. Groups on the Periodic Table Alkali Metals: Column ___________________________ very _______________________________ and explosive in ______________________________________________. Metals: 4 main characteristics of metals. It __________________________________________ electricity. Halogens: very __________________________________________, often combining with metals and Alkali Noble gas: will _________________________________________ react with ANY elements on the periodic _____________________________________________.