Lab 11.2 Spectral analysis of Light
advertisement

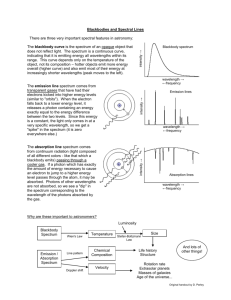
Name---------------------------- Date--------- Lab 11.2 Spectral Analysis of Fluorescent Lights Introduction I A marine ecologist ·notices that many bottom-dwelling organisms dying in a local harbor. A high school junior with an intrest in boating and ecology volunteers to help the ecologist find the cause. From mud samples taken from the bottom of the harbor, the student chemically extracts metal ions. Her quest is to see if there are any toxic metals in the mud. In the ecologist's laboratory, the student injects the metal ions into the hot flame of the atomic emission spectrometer, an instrument that can measure the wavelengths emitted by atoms. Energy from the flame excites the electrons of the metal ions and boosts them to higher energy levels from which they cascade downward in a distinctive pattern, losing their energy in the form of light. As you know from the lecture, · each type of atom produces a specific set of colors at wavelengths characteristic of that type of atom. The light emitted from the metal ions is observed and recorded after it passes through a diffraction grating. The diffraction grating spreads the light into a line spectrum. In samples from the upper layers of mud, the student observes a line spectrum with wavelengths of 563.1 nm and 615.0 nm, indicating the presence of tin. The student traces the tin to a newly marketed paint used to coat the bottoms of boats. The paint proves to be lethal to almost all bottomdwelling marine organisms and is banned from further use. So the mystery of the dying marine life is solved. In this investigation you too will be a detective, identifying a gas by its spectral lines. First, you will see how the spectrum of visible light looks when viewed through a diffraction grating spectrometer. Then will familiarize yourself with the line spectra of various elements, and the relationship between color and light wavelength. Finally, you will use this knowledge to identify the gas in a fluorescent light bulb. Pre-Lab Discussion Read the entire laboratory investigation and the relevant pages of your textbook. Then answer the questions that follow. 1. What instrument will you use to separate colors of light and analyze their wavel engt hs? _______________________ 2. When atoms are heated so that they emit light, what characteristic of their light allows you to tell one atom from another? ©Prentice-Hall, Inc. _ Spectral Analysis of Fluorescent Lights 61 Procedure - 1. View the incandescent light source with the spectrometer. In Figure 11-1 in the Observations section, sketch what you see, marking the wavelengths that define each colored region. Note the regions where the colors are brightest and dimmest. 2. Use the spectrometer to observe the light from each of the labeled gas spectrum tubes. 3. For each spectrum tube, carefully measure and list the observed wavelengths of the set of lines produced by the gas. Record the color of each line. 4. Now turn your spectrometer slit toward a fluorescent light fixture. Look carefully at each colored region of the light spectrum. You should see a line spectrum ·standing out from the full spectrum background. In addition, certain regions may be abnormally dim or missing. Measure the wavelength of the line spectrum and any dim or missing regions. Observations 1. Sketch the spectrum of incandescent white light on the blank diagram in Figure 11-1. Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Gas Type:___________________ 500nm 450nm 550nm 600 nm 650nm Critical Thinking: Analysis and Conclusions 1. What is the gas inside the fluorescent light bulb? Support your conclusion with data from Table 11-1 and with your observations of the line spectra produced by the spectrum-tube gases. 2. Why do you think that some of the reference lines given for the various gases you observed were not visible to you?